Abstract
Objectives
Noncontact mapping has been demonstrated to facilitate RF ablation of ventricular arrhythmias, but the reproducibility in the localization of endocardial exit sites during focal ventricular tachycardia (“VT”) originating from defined myocardial layers has not been systematically studied. Furthermore, it remains unclear whether noncontact mapping can distinguish between endo- and epicardial foci.
Methods
In six dogs, constant pacing was applied through octopolar needle electrodes in the left ventricle to mimic VT of subendocardial, midmyocardial (mid1; mid2) or subepicardial origin. Using noncontact mapping, the site of origin was determined for each of 50 consecutive beats of all “VTs” and the variation between respective exit sites was measured. Exit sites were reconstructed for 50 consecutive beats of each “VT” and the time span between site of origin and exit site was measured as a parameter of intramural conduction.
Results
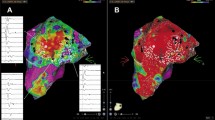
While subendocardial and midmyocardial (mid1, mid2) foci were pinpointed with a variation of ≤2 mm, a variation of 4 mm was encountered for subepicardial foci. A gradual increase in intramural conduction was evident from endocardial towards epicardial foci, with significant differences between subendocardial (4.8 ± 0.9 ms), midmyocardial (mid1 = 11.1 ± 4.6 ms; mid2 = 11.8 ± 3.5 ms) and subepicardial (16.8 ± 3.6 ms) foci (P < 0.005). Systematic differences in the morphology of virtual waveforms depending on the site of origin could not be detected.
Conclusions
Except for subepicardial foci, noncontact mapping localized focal activity in the LV with high reproducibility. In contrast to morphological parameters, the determination of intramural conduction provides a fair estimate of the depth of foci and is proposed as a novel parameter to identify a subepicardial origin.








Similar content being viewed by others
Abbreviations
- LV:
-
Left ventricle
- MEA:
-
Multi-electrode array
- Mid 1:
-
Midmyocardium 1
- Mid 2:
-
Midmyocardium 2
- NCM:
-
Noncontact mapping
- Subendo:
-
Subendocardium
- Subepi:
-
Subepicardium
- “VT”:
-
Ventricular tachycardia mimicked by ventricular pacing
References
Bartlett TG, Mitchell R, Friedman PL, Stevenson WG (1995) Histologic evolution of radiofrequency lesions in an old human myocardial infarct causing ventricular tachycardia. J Cardiovasc Electrophysiol 6:625–629
Bauer A, Schnabel PA, Schreiner KD, Becker R, Voss F, Kraft P et al (2001) Effects of propafenone on anisotropic conduction properties within the three-dimensional structure of the canine ventricular wall. Basic Res Cardiol 96:175–183
Becker R, Schoels W (2004) Ablation of atrial fibrillation: energy sources and navigation tools: a review. J Electrocardiol 37:55–62
Betts TR, Roberts PR, Allen SA, Morgan JM (2000) Radiofrequency ablation of idiopathic left ventricular tachycardia at the site of earliest activation as determined by noncontact mapping. J Cardiovasc Electrophysiol 11:1094–1101
Dorwarth U, Fiek M, Remp T, Reithmann C, Dugas M, Steinbeck G et al (2003) Radiofrequency catheter ablation: different cooled and noncooled electrode systems induce specific lesion geometries and adverse effect profiles. Pac Clin Electrophysiol 26:1438–1445
Eick OJ, Bierbaum D (2003) Tissue temperature-controlled radiofrequency ablation. Pac Clin Electrophysiol 26:725–730
Gornick CC, Adler SW, Pederson B, Hauck J, Budd J, Schweitzer J (1999) Validation of a new noncontact catheter system for electroanatomic mapping of the left ventricular endocardium. Circulation 99:829–835
Khoury DS, Berrier KL, Badruddin SM, Zoghbi WA (1998) Three-dimensional electrophysiological imaging of the intact canine left ventricle using a noncontact multielectrode cavity probe: study of sinus, paced, and spontaneous premature beats. Circulation 97:399–409
Kroll M, Kriebel T, Windhagen-Mahnert B, Franzbach B, Jux C, Zutz M et al. (2003) Origin of electrical activation within the right atrial and left ventricular walls: differentiation by electrogram characteristics using the noncontact mapping system. Pac Clin Electrophysiol 26:1970–1978
Lacroix D, Klug D, Marquié C, Kouakam C, Grandmougin D, Kacet S (2002) Identification of ventricular tachycardia of epicardial origin from unipolar potential obtained at the endocardial surface: Is it feasible? Pac Clin Electrophysiol 25:1561–1570
Scheinmann MM (1994) Patterns of catheter ablation practice in the United States: Results of the 1992 NASPE survey. Pac Clin Electrophysiol 17:873–875
Schilling RJ, Peters NS, Davies DW (1998) Simultaneous endocardial mapping in the human left ventricle using a noncontact catheter: comparison of contact and reconstructed electrograms during sinus rhythm. Circulation 98:887–898
Schilling RJ, Peters NS, Davies DW (1999) Feasibility of non-contact catheter for endocardial mapping in human ventricular tachycardia. Circulation 99:2543–2552
Schneider MAE, Ndrepepa G, Weber S, Deisenhofer I, Schoemig A, Schmitt C (2004) Influence of high-pass filtering on noncontact mapping and ablation of atrial tachycardias. Pac Clin Electrophysiol 27:38–46
Taccardi B, Arisi G, Macchi E, Baruffi S, Spaggiari S (1987) A new intracavitary probe for detecting the site of origin of ectopic ventricular beats during one cardiac cycle. Circulation 75:272–281
Thiagalingam A, Wallace EM, Boyd AC, Eipper VE, Campbell CR, Byth K et al (2004) Noncontact mapping of the left ventricle: insights from validation with transmural contact mapping. Pac Clin Electrophysiol 27:570–578
Whitwam W, Rule S, Narayan SM (2007) Noncontact mapping of small pulmonary artery potentials preceding ectopy from the right ventricular outflow tract. Heart Rhythm 4:959–963
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Frederik Voss and Dr. Alexander Bauer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Voss, F., Bauer, A., Witte, S. et al. Can noncontact mapping distinguish between endo- and epicardial foci?. Clin Res Cardiol 97, 734–741 (2008). https://doi.org/10.1007/s00392-008-0665-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-008-0665-6