Abstract
Background
Limited attention was paid to adenocarcinoma with mixed subtypes (AM) of the colon and rectum due to its low incidence. This study aims to assess the frequency and survival rates of tumors in the population.
Methods
The data were extracted from the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2019. The incidence of tumors was evaluated based on patient gender, age, race, and location. Univariate and multivariate Cox analyses were performed to identify risk factors associated with tumor survival. Additionally, a nomogram was constructed using these risk factors to predict cancer-specific survival (CSS) at 1, 2, and 3 years. Receiver operating characteristic (ROC) and calibration curves were applied to examine the model’s accuracy.
Results
The overall incidence of colorectal AM reached its highest level in 2016 (2.350 (95% CI: 2.241–2.462)). AM is more frequent in elderly patients and predominantly located in the rectum. By forest plot for multivariable Cox regression analysis, patient age, the number of regional positive lymph nodes and lymph nodes removed, tumor N/M stage, and postoperative chemotherapy were identified as independent risk indicators for CSS. Nomogram was constructed and validated as a feasible prediction model of CSS in patients with colorectal AM.
Conclusion
The presence of colorectal AM in elderly patients, particularly in the rectum, is frequent and often associated with poor prognosis. Our nomograms can offer a relatively accurate prediction of CSS of patients with AM after tumor resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to the latest cancer statistics, colorectal cancer ranked as the second most commonly diagnosed cancer in women and the third most frequently diagnosed cancer in men [1]. In 2023, approximately 52,550 patients died from colorectal cancer based on incidence from population-based cancer registries and mortality data from the National Center for Health Statistics [2]. Colorectal cancer has become the fourth leading cause of cancer-related death worldwide [1]. Colorectal cancer can be divided into adenocarcinoma, squamous cell carcinoma, and other types according to pathological classification. Adenocarcinoma accounts for 90% of all colorectal cancer cases, originating from the epithelial cells of the colorectal mucosa [3]. Classical adenocarcinoma (CA), mucinous adenocarcinoma (MAC), and signet-ring cell carcinoma (SRCC) are the three main types of adenocarcinoma [4].
Adenocarcinoma with mixed subtypes (AM) is an uncommon form of adenocarcinoma characterized by a mixture of metaplastic and conventional adenocarcinoma components [5]. Morphologically, signet-ring cellular components can be observed within AM [6, 7]. A population-based study has shown that AM was a particularly aggressive histologic subtype of colorectal cancer and had a comparable prognosis to SRCC [8]. However, due to its rarity compared to other pathological subtypes of adenocarcinoma, data on its incidence and survival rates are limited, with few studies focusing on this area. Hence, it is imperative to systematically evaluate colorectal AM incidence and survival in large populations.
The present study aimed to investigate and analyze the incidence and survival of colorectal AM based on data extracted from the Surveillance, Epidemiology, and End Results (SEER) database. The incidence of AM was analyzed based on the patient’s age, sex, race, and tumor site. In addition, a predictive nomogram was constructed to predict the survival rate of patients with colorectal AM.
Materials and methods
Data source and patient
A population-based retrospective cohort study was conducted based on Incidence-SEER Research Plus Data, 17 Registries, Nov 2021 Sub (2000–2019). Patients who met the following criteria are included: (1) Adenocarcinoma (CA, SRCC, AM, and MAC) as diagnosed by pathology. (2) Tumors in the colorectum. Patients who met the following criteria are excluded: (1) Patient age, race, and survival are unavailable. (2) Tumor size and TNM stage are missing. (3) The number of regional lymph nodes, positive lymph nodes, and lymph nodes removed is not unknown. (4) Colorectal adenocarcinoma has not been confirmed by positive pathology.
Search strategy
The present analysis included all patients diagnosed with colorectal cancer and assigned the primary site C18.2–C18.9, C19.9, and C20.9. The topography and histology of the cancer were coded using ICD-O-3 Hist/behave in cancer registries. Our analysis focused on four histologic subtypes — CA (8140/3), AM (8255/3), SRCC (8490/3), and MAC (8480/3). The following variables were extracted from the seer database: patient age, gender, race, tumor site, size, TNM stage (AJCC 6th), chemotherapy, and the number of regional lymph nodes, positive lymph nodes, and lymph nodes removed. Finally, patients with missing or unavailable variables were excluded from the analysis.
Statistical analysis
All data analyses were performed in R software version 4.4.2 (Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org/). We calculated the incidences in patients with colorectal AM between 2000 and 2019. Then, the colorectal adenocarcinoma was separated into four groups based on histology (CA, SRCC, AM, and MAC) to compare the differences in cancer overall survival (OS) and cancer-specific survival (CSS) based on the Kaplan-Meier curves and log-rank test. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic factors in OS and CSS of colorectal adenocarcinoma.
Patients with colorectal AM were extracted from the above dataset containing AM, SRCC, CA, and MAC. The predictive research was explicitly focused on CSS in colorectal AM patients, who were divided into training and validation groups in a 7:3 ratio. A forest plot was used for multivariable Cox regression analysis of patients with colorectal AM to identify independent risk factors for CSS (P < 0.05). A nomogram was constructed based on the independent risk factors to predict the 1-, 2-, and 3-year CSS rates. Calibration curves and receiver operating characteristic (ROC) curves were performed to analyze the feasibility of the nomogram for predicting CSS at 1, 2, and 3 years.
Results
Clinicopathological characteristics
Results showed that out of the 212,902 patients identified from the SEER database during 2000–2019, 928 had AM, 2536 had SRCC, 192,034 had CA, and 17,404 had MAC. The flow chart of data selection is shown in Fig. 1. The patient’s characteristics are shown in Table 1. The incidence of typical adenocarcinoma is the highest among all types of adenocarcinomas, accounting for 90.2% of cases, which surpasses that of other adenocarcinoma types by a significant margin. Among the four types of adenocarcinomas, AM has the lowest incidence at 0.4%, followed by SRCC at 1.2%. As for the comparison of clinicopathological variables, significant differences were observed between groups (P < 0.05).
Incidence and trend of AM
The incidence and trends of colorectal AM and its differences among patient subgroups were assessed in Figs. 2 and 3. The overall incidence and movement of colorectal AM had been increasing before 2017 and subsequently decreased. The highest overall incidence in the colorectum was 2.350 (95% CI: 2.241–2.462) per 100,000 person-years. Figure 3 shows the differences in tumor incidence among patients with different ages, gender, race, and tumor site. Among the older population, the incidence was higher than the young population (Fig. 3A). No essential differences in the overall incidence and trend of tumors were observed between male and female cases (Fig. 3B). The incidence was 3.682 (95% CI: 0.191–7.293) for the white population and 4.706 (95% CI: 0.703–8.868) for the black population per 100,000 person-years. Blacks had the highest incidence in the overall population (Fig. 3C). As for the site of the tumor occurrence, AM mainly occurred in the rectum. No apparent differences were observed in the incidence and trends of AM between the colon and anus (Fig. 3D).
Survival analysis between different pathological subgroups
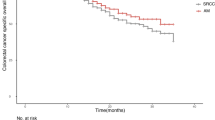
The present study employed Kaplan-Meier curves to analyze the relationship between tumor pathology and clinical outcomes such as CSS and OS. Notably, the CSS and OS of patients with CA and MAC are significantly higher than those of patients with SRCC and AM, while patients with AM and SRCC displayed similar prognoses (Fig. 4). The 1-, 2-, and 3-year OS and CSS are shown in Tables 2 and 3. In the entire population, the median OS in CA, AM, SRCC, and MAC patients were 78 months (77–78 months), 29 months (25–33 months), 22 months (20–24 months), and 63 months (61–65 months), respectively. In the population containing patients who died from colorectal cancer, the median CSS in CA, AM, SRCC, and MAC patients were 140 months (135–145 months), 27 months (22–32 months), 21 months (19–23 months), and 86 (80–93 months), respectively. Lastly, univariate and multivariate Cox analyses were performed to identify independent prognostic factors of OS and CSS in patients with colorectal CA, AM, SRCC, and MAC (Table 4). Patients with CA and MAC benefited more in terms of OS and CSS compared with those with SRCC and AM.
Construction of the nomogram for predicting CSS of colorectal AM
Patient age, tumor TNM stage, postoperative chemotherapy, and the number of regional positive lymph nodes and lymph nodes removed were identified as risk factors for CSS by Cox regression forest plots (Fig. 5). A total of 742 patients with colorectal AM were extracted to analyze and predict the CSS at 1, 2, and 3 years. Of these, 519 patients were assigned to the training group and 223 to the validation group. The specific clinicopathological features of the two groups are shown in Table 5. The Chi-square and Fisher’s exact test indicated no statistically significant difference between the training and validation groups for all variables (P > 0.05). Figure 6 shows a novel nomogram based on risk factors confirmed through multivariate Cox regression in the training group to determine the 1-, 2-, and 3-year CSS of patients with AM. In addition, the C-index value of CSS in colorectal AM was shown in Fig. 7 (training group: 1 year = 0.80, 2 years = 0.83, 3 years = 0.88; validation group: 1 year = 0.82, 2 years = 0.813, 3 years = 0.83), which also showed good discrimination in predicting the CSS of colorectal patients with AM. Calibration curves were plotted to reveal the high coherence between the nomogram-predicted and actual CSS at 1, 2, and 3 years (Fig. 8).
Discussion
According to current knowledge, there have been limited reports on the occurrence and survival rates of colorectal AM [8], and the clinicopathological features of AM remain unclear. Additionally, there is a lack of unified pathological diagnostic criteria [6, 9]. In view of this, this study aimed to address the lack of knowledge regarding the incidence rate and survival of colorectal AM.
Utilizing data from the SEER database, the study focused on the incidence rate and survival of patients with colorectal AM. An upward trend in the incidence of AM in colorectal cancer was observed between 2000 and 2017. Factors such as patient age, race, and tumor site are associated with cancer incidence, with a higher frequency observed in patients aged over 65 years and with rectal tumors. In addition, our research showed that colorectal AM and SRCC were considered to be related to poor prognosis compared to colorectal CA and MAC. And a nomogram for 1-, 2-, and 3-year CSS prediction was constructed and validated as a reliable model for patients with colorectal AM in our study.
According to our investigation, the older population is more susceptible to developing colorectal AM than their younger population. Previous studies have indicated a noteworthy increase in the frequency of colorectal adenocarcinoma among the elderly population [10,11,12], but no relevant research has been conducted on the particular difference in the incidence rate of colorectal AM between young and elderly cohorts. With regard to the predilection sites of colorectal AM, the incidence rate of rectal tumors is significantly higher than that of the colon and anus. In 2023, there were an estimated 153,020 new cases of colorectal cancer in the USA, of which 106,970 cases were tumors in the colon and 46,050 were tumors in the rectum, demonstrating a higher likelihood of colorectal tumors occurring in the rectum [2]. Due to the scarcity of literature on the particular pathological type of AM in colorectal cancer, further research is necessary to determine the incidence of AM in different subgroups.
Numerous studies have been conducted to report the clinical and pathological features of gastric AM. However, there is a dearth of the relevant literature concerning colorectal AM [13,14,15]. Since AM has been known to include signet-ring cellular components [6, 7], patients with colorectal SRCC were extracted from the database and compared with patients with colorectal AM. A population-based study carried out by Benesch M. G. K. et al. demonstrated that SRCC had a worse prognosis than conventional adenocarcinoma [16]. In line with our research, OS and CSS were significantly higher in patients with CA than those with SRCC. Additionally, there is no significant difference in OS between patients with SRCC and AM, as depicted in Fig. 4. Colorectal AM was associated with poor survival.
According to the forest plot depicting the outcomes of multivariate Cox regression analysis for CSS of colorectal AM, patient age, tumor T/N/M stage, and the number of positive regional lymph nodes and lymph nodes removed were identified as independent risk factors. The prognosis of colorectal cancer was positively associated with the removal of more than four regional lymph nodes, whereas there was no significant difference in tumor prognosis between the removal of less than three lymph nodes and no lymph node removal, which was consistent with previous studies [17, 18].
Concerning tumor T/N/M stage, the poor prognosis was easily detected, especially in patients with T4/N2/M1 stage. Bianchi G. et al. conducted a retrospective study from 2002 to 2018, involving 2652 patients with I–III stage colorectal adenocarcinoma. The study revealed that the N stage was significantly associated with lymphovascular invasion [19], which played an essential role in the survival rate of patients with colorectal cancer [19, 20]. As per the sixth edition of AJCC staging for colorectal cancer, the presence of distant metastasis in the M1 stage indicates a poorer tumor prognosis compared to those without distant metastasis [21,22,23].
The association between tumor N stage and the number of regional lymph nodes has been established, with numerous studies indicating that the number of positive lymph nodes was a crucial risk factor for tumor survival [24, 25]. The present study revealed that a more significant number of regional lymph nodes removed and fewer regional positive lymph nodes resulted in a better prognosis of tumor patients at 1, 2, and 3 years after surgery. A retrospective cohort study consisting of 2198 patients was performed by Hu et al., demonstrating that log odds of positive lymph nodes exhibited a satisfying predictive ability for patient survival, even better than tumor N stage [26]. Consistent with our research, there was a correlation between the number of lymph node resections and postoperative survival of patients [27,28,29]. Complete mesocolic excision (CME) is currently recommended as one of the surgical methods for treating colon cancer [30, 31], which involves the removal of more lymph nodes than conventional surgery, including mesenteric lymph nodes and central vascular ligation (CVL) [32]. Studies have shown that lower tumor recurrence rates and higher survival rates were found in these patients undergoing CME [33].
Retrospective studies utilizing the SEER database are subject to certain inherent limitations. While patients from the SEER database were categorized into chemotherapy and nonchemotherapy groups, specific details regarding the chemotherapy regimen were unavailable. Additionally, it is impossible to ascertain whether patients included in the study have synchronous tumors in other regions or comorbidities, which inevitably impact the postoperative prognosis of tumor patients. Finally, preoperative neoadjuvant therapy, the level of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), and specific details regarding tumor pathology are known to be associated with tumor prognosis [34,35,36]. However, none of the relevant data were considered in this study.
Conclusion
To summarize, AM is a type of colorectal cancer typically with a negative prognosis and is more prevalent among elderly individuals and in the rectum. Patients’ age, chemotherapy, tumor T/N/M stage, and the number of positive lymph nodes and lymph nodes removed are strongly linked to the OS and CSS of patients with colorectal AM. The nomogram established based on these risk factors has been developed and validated for predictive survival in colorectal AM patients. Nevertheless, further research is necessary to gain a better understanding of the clinical and pathological characteristics of this particular type of colorectal cancer.
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
References
Dekker E, Tanis P, Vleugels J, Kasi P, Wallace M (2019) Colorectal cancer. Lancet (London, England) 394:1467–1480. https://doi.org/10.1016/s0140-6736(19)32319-0
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A (2023) Colorectal cancer statistics. CA Cancer J Clin 73:233–254. https://doi.org/10.3322/caac.21772
Fleming M, Ravula S, Tatishchev S, Wang H (2012) Colorectal carcinoma: pathologic aspects. J Gastrointest Oncol 3:153–173. https://doi.org/10.3978/j.issn.2078-6891.2012.030
Ueno H, Mochizuki H, Hashiguchi Y et al (2008) Histological grading of colorectal cancer: a simple and objective method. Ann Surg 247:811–818. https://doi.org/10.1097/SLA.0b013e318167580f
Kushima R (2021) The updated WHO classification of digestive system tumours—gastric adenocarcinoma and dysplasia. Pathologe 43:8–15. https://doi.org/10.1007/s00292-021-01023-7
Nagtegaal ID, Odze RD, Klimstra D et al (2019) WHO classification of tumours of the digestive system. Histopathology 76:182–188. https://doi.org/10.1111/his.13975
Ahadi M, Sokolova A, Brown I, Chou A, Gill AJ (2019) World Health Organization classification of appendiceal, colorectal and anal canal tumours: an update and critical assessment. Pathology 53:454–461. https://doi.org/10.1016/j.pathol.2020.10.010
Sheng H, Wei X, Mao M et al (2019) Adenocarcinoma with mixed subtypes is a rare but aggressive histologic subtype in colorectal cancer. BMC Cancer 19:1071. https://doi.org/10.1186/s12885-019-6245-5
Meng NL, Wang YK, Wang HL, Zhou JL, Wang SN (2022) Research on the histological features and pathological types of gastric adenocarcinoma with mucinous differentiation. Front Med (Lausanne) 9:829702. https://doi.org/10.3389/fmed.2022.829702
Nso N, Nyabera A, Nassar M et al (2023) Incidence and risk factors of cardiovascular mortality in patients with gastrointestinal adenocarcinoma. PLoS One 18:e0262013. https://doi.org/10.1371/journal.pone.0262013
Möller L, Wellmann I, Stang A, Kajüter H (2023) The epidemiology of colorectal cancer in younger and older patients. Dtsch Arztebl Int 120(16):277. https://doi.org/10.3238/arztebl.m2023.0041
Saraiva MR, Rosa I, Claro I (2023) Early-onset colorectal cancer: a review of current knowledge. World J Gastroenterol 29:1289–1303. https://doi.org/10.3748/wjg.v29.i8.1289
Koemans W, Luijten J, van der Kaaij R et al (2020) The metastatic pattern of intestinal and diffuse type gastric carcinoma — a Dutch national cohort study. Cancer Epidemiol 69:101846. https://doi.org/10.1016/j.canep.2020.101846
van der Kaaij R, Koemans W, van Putten M et al (2020) A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur J Cancer 130:23–31. https://doi.org/10.1016/j.ejca.2020.02.017
Pernot S, Terme M, Radosevic-Robin N et al (2020) Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer 23:73–81. https://doi.org/10.1007/s10120-019-00983-3
Benesch M, Mathieson A, O’Brien S (2023) Effects of tumor localization, age, and stage on the outcomes of gastric and colorectal signet ring cell adenocarcinomas. Cancers 15. https://doi.org/10.3390/cancers15030714
Christou N, Meyer J, Toso C, Ris F, Buchs N (2019) Lateral lymph node dissection for low rectal cancer: is it necessary? World J Gastroenterol 25:4294–4299. https://doi.org/10.3748/wjg.v25.i31.4294
Ishizaki T, Mazaki J, Kasahara K et al (2023) Robotic versus laparoscopic approach for minimally invasive lateral pelvic lymph node dissection of advanced lower rectal cancer: a retrospective study comparing short-term outcomes. Tech Coloproctol 1–9. https://doi.org/10.1007/s10151-023-02818-x
Bianchi G, Annicchiarico A, Morini A et al (2021) Three distinct outcomes in patients with colorectal adenocarcinoma and lymphovascular invasion: the good, the bad, and the ugly. Int J Colorectal Dis 36:2671–2681. https://doi.org/10.1007/s00384-021-04004-7
Lin Z, Zheng Y, Yang J et al (2023) Prognostic analysis of lymphovascular invasion in stages I–III colorectal cancer: a retrospective study based on propensity score match. Am J Clin Oncol 10-1097. https://doi.org/10.1097/coc.0000000000001015
Kröning H, Göhler T, Decker T et al (2023) Effectiveness, safety and quality of life of trifluridine/tipiracil in pretreated patients with metastatic colorectal cancer: real-world data from the noninterventional TACTIC study in Germany. Int J Cancer. https://doi.org/10.1002/ijc.34603
Lehtomäki K, Soveri L, Osterlund E et al (2023) Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study). J Clin Med 12:3541. https://doi.org/10.3390/jcm12103541
Vogl T, Hammann L, Adwan H (2023) Transvenous pulmonary chemoembolization and optional microwave ablation for colorectal lung metastases. J Clin Med 12:3394. https://doi.org/10.3390/jcm12103394
Baqar AR, Wilkins S, Wang W, Oliva K, McMurrick P (2020) Log odds of positive lymph nodes is prognostically equivalent to lymph node ratio in non-metastatic colon cancer. BMC Cancer 20:762. https://doi.org/10.1186/s12885-020-07260-y
Xiong X, Wang C, Cao J, Gao Z, Ye Y (2023) Lymph node metastasis in T1–2 colorectal cancer: a population-based study. Int J Colorectal Dis 38:94. https://doi.org/10.1007/s00384-023-04386-w
Hu X, Jiang L, Wu J, Mao W (2023) Prognostic value of log odds of positive lymph nodes, lymph node ratio, and N stage in patients with colorectal signet ring cell carcinoma: a retrospective cohort study. Front Surg 9:1019454. https://doi.org/10.3389/fsurg.2022.1019454
Shulman LN, Browner AE, Palis BE et al (2019) Compliance with cancer quality measures over time and their association with survival outcomes: the commission on cancer’s experience with the quality measure requiring at least 12 regional lymph nodes to be removed and analyzed with colon cancer resections. Ann Surg Oncol 26:1613–1621. https://doi.org/10.1245/s10434-019-07323-w
Anania G, Davies R, Bagolini F et al (2019) Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctol 25:1099–1113. https://doi.org/10.1007/s10151-021-02471-2
Longchamp G, Meyer J, Christou N et al (2020) Total mesorectal excision with and without lateral lymph node dissection: a systematic review of the literature. Int J Colorectal Dis 35:1183–1192. https://doi.org/10.1007/s00384-020-03623-w
Ning FL, Pei JP, Zhang NN et al (2020) Harvest of at least 18 lymph nodes is associated with improved survival in patients with pN0 colon cancer: a retrospective cohort study. J Cancer Res Clin Oncol 146:2117–2133. https://doi.org/10.1007/s00432-020-03212-y
Son GM, Lee IY, Lee YS et al (2021) Is laparoscopic complete mesocolic excision and central vascular ligation really necessary for all patients with right-sided colon cancer? Ann Coloproctol 37:434–444. https://doi.org/10.3393/ac.2021.00955.0136
Killeen S, Mannion M, Devaney A, Winter D (2014) Complete mesocolic resection and extended lymphadenectomy for colon cancer: a systematic review. Colorectal Dis 16:577–594. https://doi.org/10.1111/codi.12616
Magistro C, Bertoglio CL, Giani A et al (2022) Laparoscopic complete mesocolic excision versus conventional resection for right-sided colon cancer: a propensity score matching analysis of short-term outcomes. Surg Endosc 36:3049–3058. https://doi.org/10.1007/s00464-021-08601-z
Murthy V, Maitre P, Singh M et al (2023) Study protocol of the Bladder Adjuvant RadioTherapy (BART) trial: a randomised phase III trial of adjuvant radiotherapy following cystectomy in bladder cancer. Clin Oncol. https://doi.org/10.1016/j.clon.2023.04.010
Lehtomäki K, Mustonen H, Kellokumpu-Lehtinen PL et al (2021) Lead time and prognostic role of serum CEA, CA19–9, IL-6, CRP, and YKL-40 after adjuvant chemotherapy in colorectal cancer. Cancers (Basel) 13:3892. https://doi.org/10.3390/cancers13153892
Kodama H, Masuishi T, Wakabayashi M et al (2023) Differential efficacy of targeted monoclonal antibodies in left-sided colon and rectal metastatic cancers. Clin Colorectal Cancer. https://doi.org/10.1016/j.clcc.2023.05.002
Author information
Authors and Affiliations
Contributions
Fan Zhang, Boqi Xu, and Shan Tong were responsible for the design of the study, statistical analysis, and drafting and revising of the manuscript; Yao Peng, Boqi Xu, and Zhongqi Mao provided critical comments and review of the manuscript; Fan Zhang, Shan Tong, and Zhongqi Mao designed and supervised the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Consent for publication
All patients consented to the publication of the results of this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fan Zhang and Boqi Xu contributed equally to this work and should be regarded as the first co-authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, F., Xu, B., Peng, Y. et al. Incidence and survival of adenocarcinoma with mixed subtypes in patients with colorectal cancer. Int J Colorectal Dis 38, 215 (2023). https://doi.org/10.1007/s00384-023-04508-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04508-4