Abstract
Purpose
Tumour-associated macrophages have been shown to promote proliferation, angiogenesis and metastasis in several carcinomas. The effect on colon cancer has not yet been clarified. Furthermore, Kupffer cells in the liver might initiate the formation of metastases by directly binding tumour cells.
Methods
An orthotopic syngeneic mouse model of colon cancer as well as a liver metastases model has been studied, using murine CT-26 colon cancer cells in Balb/c-mice. Macrophages were depleted in both models by clodronate liposomes. Tumour sizes and metastases were determined using 7-Tesla MRI. The macrophage and vascular density in the orthotopic tumours as well as the Kupffer cell density in the livers were evaluated using immunohistochemistry.
Results
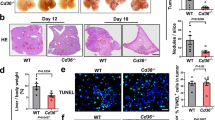
Animals in the macrophage-depleted group displayed significantly smaller primary tumours (37 ± 20 mm3) compared to the control group (683 ± 389 mm3, p = 0.0072). None of the mice in the depleted group showed liver or peritoneal metastases, whereas four of six control mice displayed liver and five out of six mice peritoneal metastases. The vascular density was significantly lower in the macrophage-depleted group (p = 0.0043). In the liver metastases model, animals of the Kupffer cell-depleted group (14.3 ± 7.7) showed significantly less liver metastases than mice of the two control groups (PBS liposomes, 118.5 ± 28.2, p = 0.0117; NaCl, 81.7 ± 23.2, p = 0.0266). The number of liver metastases correlated directly with the Kupffer cell density (p = 0.0221).
Conclusion
Macrophages promote tumour growth, angiogenesis and metastases in this orthotopic syngeneic mouse model. Kupffer cells enhance the formation of metastases in the liver.










Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW (2005) Colorectal cancer. Lancet 365(9454):153–165
Cirocchi R, Trastulli S, Boselli C, Montedori A, Cavaliere D, Parisi A, Noya G, Abraha I (2012) Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev 6, CD006317
Renehan AG, Egger M, Saunders MP, O'Dwyer ST (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 324(7341):813
Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor-associated macrophages. Immunol Today 13(7):265–270
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Goerdt S, Orfanos CE (1999) Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10(2):137–142
Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66(1):1–9
Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P (2006) Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211(6–8):487–501
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Sica A, Schioppa T, Mantovani A, Allavena P (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42(6):717–727
Mantovani A (1994) Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Investig 71:5–16
Graves DT, Jiang YL, Williamson MJ, Valente AJ (1989) Identification of monocyte chemotactic activity produced by malignant cells. Science 245(4925):1490–1493
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196(3):254–265
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Lee AH, Happerfield LC, Bobrow LG, Millis RR (1997) Angiogenesis and inflammation in invasive carcinoma of the breast. J Clin Pathol 50(8):669–673
Salvesen HB, Akslen LA (1999) Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer 84(5):538–543
Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y (2000) Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7(7):263–269
Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17(3):445–451
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, Suzuki N, Inoue M, Soma G, Nagasue N (2003) The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 23(6D):5015–5022
Ma J, Liu L, Che G, Yu N, Dai F, You Z (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10:112
Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R (2007) High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 13(5):1472–1479
Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, Zeng YX, Zhang XS (2010) The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 8:13
Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A (2007) TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer 7:156
Bailey C, Negus R, Morris A, Ziprin P, Goldin R, Allavena P, Peck D, Darzi A (2007) Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis 24(2):121–130
Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW (2002) Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res 62(23):7042–7049
Braet F, Nagatsuma K, Saito M, Soon L, Wisse E, Matsuura T (2007) The hepatic sinusoidal endothelial lining and colorectal liver metastases. World J Gastroenterol 13(6):821–825
Paschos KA, Majeed AW, Bird NC (2010) Role of Kupffer cells in the outgrowth of colorectal cancer liver metastases. Hepatol Res 40(1):83–94
Bayon LG, Izquierdo MA, Sirovich I, van Rooijen N, Beelen RH, Meijer S (1996) Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology 23(5):1224–1231
Heuff G, Oldenburg HS, Boutkan H, Visser JJ, Beelen RH, Van Rooijen N, Dijkstra CD, Meyer S (1993) Enhanced tumour growth in the rat liver after selective elimination of Kupffer cells. Cancer Immunol Immunother 37(2):125–130
Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, Wisse E, Braet F (2004) Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer 112(5):793–802
Gangopadhyay A, Lazure DA, Thomas P (1998) Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis 16(8):703–712
Aarons CB, Bajenova O, Andrews C, Heydrick S, Bushell KN, Reed KL, Thomas P, Becker JM, Stucchi AF (2007) Carcinoembryonic antigen-stimulated THP-1 macrophages activate endothelial cells and increase cell–cell adhesion of colorectal cancer cells. Clin Exp Metastasis 24(3):201–209
Kruskal JB, Azouz A, Korideck H, El-Hallak M, Robson SC, Thomas P, Goldberg SN (2007) Hepatic colorectal cancer metastases: imaging initial steps of formation in mice. Radiology 243(3):703–711
Corbett TH, Griswold DP Jr, Roberts BJ, Peckham JC, Schabel FM Jr (1975) Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res 35(9):2434–2439
Van Rooijen N, Sanders A (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174(1–2):83–93
van Rooijen N, Hendrikx E (2010) Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 605:189–203
Partecke IL, Kaeding A, Sendler M, Albers N, Kuhn JP, Speerforck S, Roese S, Seubert F, Diedrich S, Kuehn S, Weiss UF, Mayerle J, Lerch MM, Hadlich S, Hosten N, Heidecke CD, Puls R, von Bernstorff W (2011) In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer 11:40
Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schafer R, Stanley ER, Abraham D (2004) Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res 64(15):5378–5384
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14(2):425–431
Lewis CE, Leek R, Harris A, McGee JO (1995) Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol 57(5):747–751
Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, Meijer S, van der Sijp JR, Beelen RH, van Egmond M (2005) Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol 207(2):147–155
Gjoen T, Seljelid R, Kolset SO (1989) Binding of metastatic colon carcinoma cells to liver macrophages. J Leukoc Biol 45(4):362–369
Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P (2002) Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res 62(19):5393–5398
Kan Z, Ivancev K, Lunderquist A, McCuskey PA, McCuskey RS, Wallace S (1995) In vivo microscopy of hepatic metastases: dynamic observation of tumor cell invasion and interaction with Kupffer cells. Hepatology 21(2):487–494
Griffini P, Smorenburg SM, Verbeek FJ, van Noorden CJ (1997) Three-dimensional reconstruction of colon carcinoma metastases in liver. J Microsc 187(Pt 1):12–21
Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R (2006) Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 116(8):2132–2141
Acknowledgments
The study was supported by the German Federal Ministry of Education and Research BMBF, grant no. 0314107.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kruse, J., von Bernstorff, W., Evert, K. et al. Macrophages promote tumour growth and liver metastasis in an orthotopic syngeneic mouse model of colon cancer. Int J Colorectal Dis 28, 1337–1349 (2013). https://doi.org/10.1007/s00384-013-1703-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1703-z