Abstract
Aim
The aim of this study was to evaluate temporal trends in treatment and outcome in rectal cancer diagnosed during 1980–2004 at Levanger Hospital.
Materials and methods
A protocol for prospective registration of rectal cancer treated with total mesorectal excision including operative strategy, radiotherapy and surveillance was established at Levanger Hospital in 1980. In this study, all rectal cancer patients treated during 1980–2004 were included.
Results
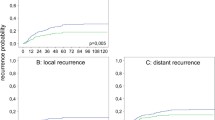
More patients received preoperative radiotherapy during 2000–2004, but otherwise there were no significant differences in presentation or treatment during 1980–2004. The 5-year local recurrence rate after resection with curative intent was 4.5% (0–9.7), 18.7% (10.3–27.1) and 2.2% (0–6.7) in 1980–1989, 1990–1999 and 2000–2004 (p = 0.006), respectively. Out of a total of 23 cases of local recurrence, treatment guidelines, mainly with regard to radiotherapy, were violated in 19 cases. The 5-year overall survival after resection with curative intent was 65% (95% confidence interval [CI] 55–76) during 1980–1989, 58% (49–68) in 1990–1999 and 71% (59–83) in 2000–2004 (n.s). The 5-year relative survival was 83% (95% CI 69–95) during 1980–1989, 71% (59–81) in 1990–1999 and 84% (69–98) in 2000–2004 (n.s).
Conclusion
Rectal cancer patients experienced excellent outcomes in the period 1980–1989 and 2000–2004. Due to violations of treatment guidelines, the rate of local recurrence was much too high in the period 1990–1999. This article illustrates the importance of continuous quality assurance in the treatment of rectal cancer to maintain optimized outcomes for the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total mesorectal excision (TME) as described by Heald et al. in 1982 has [1] become the gold standard of rectal cancer surgery. When performed meticulously, it yields low rates of local recurrence (LR) and improved survival [1]. In Norway, a national rectal cancer project was launched in 1993 in order to improve outcome for rectal cancer patients. Of several interventions, TME was introduced as the preferred surgical technique and an educational programme, which included training courses and master classes, was implemented throughout Norway [2]. As a result, the risk of LR was decreased by 50% [3].
At Levanger Hospital, the modern principles of rectal cancer surgery were introduced in 1980, and a prospective protocol for operative strategy, radiotherapy and surveillance was established. Although excellent results were reported [4], an offer to participate in the TME educational programme was rejected by Levanger Hospital because it was a common belief among our staff that we had mastered this technique. However, the first biannual report from the Cancer Registry of Norway in 1997 revealed an unacceptably high rate of LR at Levanger hospital, and actions had to be taken. The protocol was regarded as sufficient and left unchanged, but the focus on adequate preoperative assessment to assign the right patients to neoadjuvant therapy was increased. In addition, the team of surgeons who performed TME surgery was strengthened by having specialists in colorectal surgery present during operation. In this study, a complete cohort of patients who were treated for rectal cancer at Levanger Hospital during 1980–2004 was analyzed to assess temporal trends in treatment and oncologic outcomes. We examined every case of LR in this time period in search of possible protocol violations.
Patients and methods
A complete cohort was assured by using data from the Norwegian Cancer Registry. The hospital health records of all patients treated for rectal cancer at Levanger Hospital from 1980 to the end of 2004 were reviewed. The patients were assigned into three separate periods, which depended on their date of surgery: 1980–1989, 1990–1999 and 2000–2004. The hospital served a defined patient catchment area with a population that increased slightly from 85,741 in 1980 to 88,858 in 2004.
The upper limit for rectal cancer was defined as 15 cm from the anal verge measured on rigid proctoscopy. The surgery was performed by sharp dissection under visual guidance in the avascular plane surrounding the mesorectal fascia. A major resection with curative intent implied resection of the tumour bearing segment of rectum with no signs of metastases on preoperative investigations or by intraoperative examination, but included patients with microscopically involved margin and intraoperative perforations. The operation was considered curative if a microscopically free margin was confirmed and there was no bowel perforation. Circumferential resection margin (CRM) was defined as the shortest distance from the periphery of the tumour or tumour deposits to the resection margin. Residual tumour stage (R stage) was registered where no residual tumour locally was classified as R0 resection, microscopically involved margin as R1 resection and macroscopically residual tumour as R2 resection. To assign cancer stage we used the TNM Classification of Malignant Tumours, sixth edition [5]. A histological examination was missing in 17 patients, of which 12 had received best supportive care without any operation and five had received nonresective procedures.
The staff of specialist surgeons was stable and increased over the past 25 years. During the years 1990–1999, many undergraduates were responsible for the preoperative examinations, although they performed the surgical procedures together with a specialist in gastrointestinal surgery. The responsibility for treatment of rectal cancer was dispersed on more persons than during 1980–1989. Since 2000, rectal cancer surgery was no longer required for surgeons specializing in general surgery, and more dedicated teams were responsible for the preoperative examinations and the treatment.
During the operation, the routine was 5 cm distal margin for tumours in the upper and middle rectum, but a 2-cm margin was accepted in lower tumours in order to avoid abdominoperineal resection.
The clinical follow-up programme after resection with curative intent was 5 years (range of follow-up 0–28.7 years), and the median follow-up with regard to survival was 9.4 years (range 5.0–28.7). The surveillance programme was principally the same for all 25 years, based on symptoms, clinical examination including proctoscopy, measure of carcinoembryonic antigen at every visit, chest radiography and colonoscopy at intervals. Liver ultrasound and CT scan were performed at intervals as these modalities became available.
LR was defined as recurrent disease in the pelvis or the perineum, regardless of whether it was distant metastasis or not. Mortality data were collected from the hospital patient administrative system. Postoperative mortality was defined as all deaths within 30 days after laparotomy or during the same hospital stay, regardless of time. Overall survival was estimated by inclusion of death from any cause. Relative survival was defined as the ratio of observed survival in rectal cancer patients to the expected survival of the general population of Norway.
Perioperative radiotherapy and operative strategy
Preoperative radiotherapy was recommended for patients who had been diagnosed with locally advanced tumours throughout the period, initially given as 46 Gy, and in later years as 2 Gy × 25 with concomitant tumour-sensitizing 5-Fluorouracil. In 1980–1999, before the era of MRI, a tumour was considered locally advanced when it was fixed in the pelvis, or if it could not be moved in two planes at the preoperative examination. The surgery was performed according to the principles described by Bjerkeset and Edna [4]. Postoperative radiotherapy was recommended in cases of an R1 resection or perforation.
Statistical methods
Two by two tables were analyzed using the unconditional z-pooled test, which is the unconditional version of Pearson’s χ 2 test [6]. The exact Cochran–Armitage test was used for testing trends in proportions. The medians of three samples were analyzed using the Kruskal–Wallis test. To analyze the association between the period of treatment as an explanatory variable and the dependent variables tumour stage, ASA score and the number of specialists in colorectal surgery, we used a proportional odds logistic regression model, also called ordinal logit model, which has been recommended by Agresti [7]. Kaplan–Meier survivor functions, with corresponding estimates and 95% confidence intervals (CI), were calculated. The survival functions were compared using the log-rank test.
Relative survival was estimated using actuarial methods and analyzed with STATA [8]. Significance tests of excess mortality were done using a full likelihood approach. Norwegian population survival probabilities for every year from 1980, by sex and age, were downloaded from the Human Mortality Database [9]. Data were not available for 2009, and according to standard practice, we made the assumption that the probabilities for 2009 were the same as for 2008.
Two-sided p values <0.05 were considered significant. The analyses were performed using SPSS 15.0, STATA 10.0 and StatXact 8.0.
The study was approved by the Regional Committee of Ethics and performed according to the Helsinki declaration.
Results
A total of 394 patients, 247 males and 147 females, were treated between 1980 and 2004. The median age at diagnosis was 69.9 years (range 37–90) during 1980–1989 and 70.1 years in 1990–1999 and 2000–2004 (range 35–93 and 44–91, respectively).
Location
A total of 125 tumours (32%) were located 12–15 cm from the anal verge, 175 (44%) were 6–11 cm from the anal verge and 94 (24%) were 0–5 cm from the anal verge. The percentage of patients with a low rectal cancer was the same during the three periods studied.
Presentation
The type of presentation by time period is shown in Table 1. The number of patients admitted as emergency cases with either obstruction or spontaneous tumour perforation was 4% (5/127) during 1980–1989, 6% (10/177) in 1990–1999 and 4% (4/90) in 2000–2004. The stage at diagnosis was similar for all three periods (Table 2). Poor differentiation was reported in 13% of all rectal cancer cases in1980–1989 and 14% in both 1990–1999 and 2000–2004.
Treatment
Type of treatment in relation to time period is shown in Table 3. Resection with curative intent was performed in 63.8% (81/127) of all rectal cancer cases during 1980–1989, 64.9% (115/177) in 1990–1999, and 62.2% (56/90) in 2000–2004 (n.s.). For patients who had a resection with curative intent, radiotherapy was given preoperatively or postoperatively as presented in Table 4. Significantly more patients received preoperative radiotherapy during 2000–2004.
The rate of resections with curative intent performed with a specialist in colorectal surgery present increased significantly from the first to the last time periods (Table 5). A surgical trainee performed the operation in 37% (30/81) of all cases during 1980–1989, 44% (51/115) in 1990–1999 and 12.5% (7/56) in 2000–2004 (p = 0.011).
The median operative time in operations with curative intent was 200 min (range 125–410) during 1980–1989, 165 min (84–370) during 1990–1999 and 150 min (67–310) during the last period (p = 0.002).
The median blood loss during operations with curative intent was 1,200 ml (300–9,000) during 1980–1989, 900 ml (200–10,000) during 1990–1999 and 650 ml (50–3,000) during the last period (p < 0.001).
The proportion with distal resection margin of 2 cm or more in operations with curative intent was 68% (55/81) during 1980–1989, 70% (81/115) during 1990–1999 and 84% (47/56), during 2000–2004 (p = 0.056).
Sphincter-sparing surgery was performed in 64% (52/81) of resections with curative intent during 1980–1989, 73% (84/115) in 1990–1999 and 66% (37/56) in 2000–2004. Anastomotic leakage was diagnosed in 10% (5/52) during 1980–1989, 6% (5/84) in 1990–1999 and 8% (3/37) in 2000–2004.
Postoperative mortality
After resection with curative intent, the postoperative mortality rate was 7.4% (6/81) during 1980–1989, 4.3% (5/115) in 1990–1999 and 3.6% (2/56) in 2000–2004 (p = 0.34). For patients treated with palliative intent, the corresponding numbers were 8.3% (2/24), 19.4% (7/36) and 0% (0/20). The overall postoperative mortality was 24% in patients presenting with spontaneous perforation or bowel obstruction.
Local recurrence
The 5-year estimated LR rate after resection with curative intent was 4.5% (0–9.7), 18.7% (10.3–27.1) and 2.2% (0–6.7) in 1980–1989, 1990–1999 and 2000–2004 (p = 0.006), respectively. Out of 11 resections with curative intent, classified as R1 resections, ten patients developed LR (Table 6). After curative resection with a distal clearance of less than 2 cm, LR developed in 11% (10/92) compared to only 3% (4/138) when the distal clearance was >2 cm (p = 0.014). Out of ten patients with R1 resections and four patients with intraoperative tumour perforation who later developed LR, only one patient received postoperative RT. Radiotherapy was only given to one of the 23 patients who later developed an LR. When no obvious risk factor was present (T1-3 cancer, no perforation, no R1 resection or distal clearance >2 cm), only four patients developed an LR.
Long-term survival
For all stages together, the estimated 5-year overall survival was 48% (95% CI 40–58) during 1980–1989, 40% (33–48) in 1990–1999 and 50% (40–61) in 2000–2004 (n.s). The corresponding estimated 5-year overall survival after resection with curative intent was 65% (95% CI 55–76) during 1980–1989, 58% (49–68) in 1990–1999 and 71% (59–83) in 2000–2004 (n.s). For all stages together, the estimated 5-year relative survival was 63% (95% CI 51–73) during 1980–1989, 50% (41–58) in 1990–1999 and 59% (46–71) in 2000–2004 (n.s.). The corresponding estimated 5-year relative survival after resection with curative intent was 83% (95% CI 69–95) during 1980–1989, 71% (59–81) in 1990–1999 and 84% (68–97) in 2000–2004 (n.s).
Discussion
Excellent overall survival, relative survival and LR rates were achieved in 1980–1989 and 2000–2004. However, during 1990–1999, the LR rate was unacceptably high and survival was correspondingly low. In almost all cases of LR, violation of treatment guidelines could be identified.
This paper presents one of the longest running experiences with TME for rectal cancer. A complete cohort of patients was obtained, thereby avoiding selection bias. A protocol for treatment and surveillance was established in 1980 and was unchanged throughout the study period.
Although the protocol was implemented for prospective registration, this study still has the limitations of a retrospective study in that the data were analyzed retrospectively. This is a minor concern considering that overall survival and relative survival are robust parameters [10], although this might have led to underestimation of LR rates. For instance, old and fragile patients received less follow-up, which could result in LR being undisclosed. However, half of all cases with LR were diagnosed in patients older than 75 years.
The results were significantly worse during 1990–1999, as can be seen by the estimated 5-year LR rates: 4.5% during 1980–1989, 18.7% in 1990–1999 and 2.2% in 2000–2004 after resections with curative intent. In order to understand why LR occurred, all cases of LR were analyzed with particular emphasis on whether the protocol outlined in 1980 had been followed. Preoperative radiotherapy was recommended in locally advanced cases to achieve R0 resections. However, none of the four patients with a stage T4 cancer who later developed LR received preoperative radiotherapy. During 2000–2004, significantly more patients received preoperative radiotherapy, and excellent results concerning LR were achieved.
The CRM is a strong predictor of LR, distant metastasis and survival [11–13] in rectal cancer. The main reason why TME has been so successful in lowering LR rates compared to traditional rectal resections is its ability to achieve R0 resections [14]. In a recent publication [15], a CRM <2 mm was associated with poorer prognosis. In this study, we found that in a total of 11 R1 resections with curative intent, out of which nine where operated on during 1990–1999, ten patients later developed LR. Only one of these ten patients received postoperative radiotherapy. Although recommended in our protocol, Marijnen et al. [16] found little evidence to support postoperative radiotherapy as this was of no benefit for patients receiving non-radical resections. On the other hand, preoperative radiotherapy was effective in cases of narrow margins (1.1–2 mm) and wider margins. Knowing this, selecting patients at risk for R1 resections for preoperative radiotherapy to achieve R0 resections is far more important than offering postoperative radiotherapy to patients with microscopically involved margin.
Intraoperative perforation during resection of rectal cancer increases the LR rate and reduces survival [17, 18]. Although this remains to be proven effective in this setting [17], postoperative radiotherapy was recommended in our protocol and has since been implemented in both Norwegian and American guidelines [19, 20]. There is no upper age limit for adjuvant radiotherapy in Norway, but individual considerations must be taken for those aged above 75 years as radiotherapy substantially increases the risk of death from causes unrelated to rectal cancer in this age group [21]. Out of four patients with tumour perforation, three did not receive postoperative radiotherapy, all aged above 80 years.
Although there is conflicting evidence concerning how long the distal margin should be in resections of rectal cancers, many researchers [20, 22] recommend a distal clearance of >2 cm in AR, which is recommended in our protocol. The rationale underlying this is the finding that intramural spread from rectal cancers >1 cm from the primary lesion is uncommon [23] and even more so if the patient has been subject to preoperative RT [24]. In the present study, significantly more patients developed LR with a distal clearance of less than 2 cm. Furthermore, none of these patients had received neoadjuvant RT. In a total of 15 patients with distal clearance <2 cm and LR, 13 underwent operation during 1990–1999. In surgery of low rectal cancers, there is often a dilemma concerning oncologic radicality and the avoidance of stomas. This study supports the view that a distal clearance of at least 2 cm should be given priority and in cases where this is hard to achieve due to sphincter-preserving surgery, preoperative RT should be considered.
The number of specialists in colorectal surgery present at operation increased during the years of the present study. After the year 2000, rectal cancer surgery was no longer required for surgeons who are specialists in general surgery in Norway, and the number of operations performed by a trainee was significantly reduced. The current policy at Levanger Hospital is that rectal cancer resections should be performed by or under the supervision of a specialist surgeon and having two specialists present is recommended. Borowski et al. [25] found no difference in anastomotic leak rates, operative mortality or survival between unsupervised trainees, supervised trainees and consultants. We still believe that the introduction of more competent teams performing the surgery has contributed to better results during 2000–2004. The better results during 1980–1989 compared to 1990–1999 are perhaps dependent on there being far more experienced trainees working at the hospital during the first period. During the 1980s, the trainees were more experienced in general surgery (6–11 years experience) than the surgeons in the 1990s (3–6 years experience).
Preoperative radiotherapy at biologically effective doses ≥30 Gy has been shown to reduce the risk of LR and death from rectal cancer, and postoperative radiotherapy has been shown to reduce the risk of LR [21]. Preoperative chemoradiation is even more effective in lowering the LR rate, and an additional effect on survival is possible [26]. At Levanger Hospital, adjuvant radiotherapy has been recommended since 1980, but was barely used during 1990–1999, which may explain why the rate of LR was high and survival correspondingly low during these years. For the years 2000–2004, when the LR rate and survival were excellent, 21% received preoperative radiotherapy. If the quality of surgery performed was evaluated by operative time, blood loss, the proportion with sphincter-sparing surgery, by the proportion without postoperative anastomotic leakage and by the proportion with a resection margin of at least 2 cm, the quality of the surgery performed for curative intent did not seem to be inferior during 1990–1999 compared with 1980–1989. Even if experienced surgeons assisted the many surgical trainees during the operations, and the operations were adequate, the ultimate outcome was inferior for some patients during 1990–1999, and this was probably mainly due to a lack of referral for radiotherapy. In many cases, the preoperative judgment concerning radiotherapy failed during 1990–1999. From year 2000, dedicated teams took care of preoperative evaluation and surgery, and the introduction of preoperative MRI for all patients with cancer of the rectum from this time on, made it possible with a much better and objective selection of those who needed preoperative radiotherapy.
In the present study, the estimated 5-year overall survival after resection with curative intent was 65% (95% CI 55–76) during 1980–1989, 58% (49–68) in 1990–1999 and 71% (59–83) in 2000–2004 (n.s). A higher rate of LR during 1990–1999 was accompanied by a lower survival rate. The negative prognostic effect of LR on survival is well documented [2].
Conclusions
Excellent results were seen during the 1980s due to the implementation of modern principles of rectal cancer treatment at Levanger Hospital in 1980. However, reports from the national rectal cancer registry revealed poor results for patients treated in Levanger during the 1990s, and the current paper discloses that violations of the treatment guidelines, particularly with respect to radiotherapy for patients with advanced stages, had serious effects on patient prognosis during these years. Actions were taken to improve compliance regarding treatment guidelines for rectal cancer and to strengthen the surgical team which took care of the patients preoperatively as well as performed the TME surgery. During 2000–2004, the results were once again excellent. The present study illustrates that although treatment guidelines and surgical technique may be adequate, continuous focus on quality assurance and the collective efforts of the members of the multidisciplinary team are mandatory to maintain optimized outcomes for rectal cancer patients.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69(10):613–616
Wibe A, Moller B, Norstein J et al (2002) A national strategic change in treatment policy for rectal cancer—implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 45(7):857–866
Wibe A, Carlsen E, Dahl O et al (2006) Nationwide quality assurance of rectal cancer treatment. Colorectal Dis 8(3):224–229
Bjerkeset T, Edna TH (1996) Rectal cancer: the influence of type of operation on local recurrence and survival. Eur J Surg 162(8):643–648
Sobin L, Wittekind C (2002) TNM classification of malignant tumors. John Wiley, New York
Lydersen S, Fagerland MW, Laake P (2009) Recommended tests for association in 2 × 2 tables. Stat Med 28(7):1159–1175
Agresti A (2007) An introduction to categorical data analysis. Wiley-Interscience, Hoboken
Dickman PW, Sloggett A, Hills M, Hakulinen T (2004) Regression models for relative survival. Stat Med 23(1):51–64
Human Mortality Database. http://www.mortality.org/(2008)
Platell CF, Semmens JB (2004) Review of survival curves for colorectal cancer. Dis Colon Rectum 47(12):2070–2075
Nagtegaal ID, Marijnen CA, Kranenbarg EK, Van De Velde CJ, van Krieken JH (2002) Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 26(3):350–357
Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2(8514):996–999
Wibe A, Rendedal PR, Svensson E et al (2002) Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 89(3):327–334
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK (1998) Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 133(8):894–899
Bernstein TE, Endreseth BH, Romundstad P, Wibe A (2009) Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg 96(11):1348–1357
Marijnen CA, Nagtegaal ID, Kapiteijn E et al (2003) Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys 55(5):1311–1320
Eriksen MT, Wibe A, Syse A, Haffner J, Wiig JN (2004) Inadvertent perforation during rectal cancer resection in Norway. Br J Surg 91(2):210–216
Jorgren F, Johansson R, Damber L, Lindmark G (2010) Oncological outcome after incidental perforation in radical rectal cancer surgery. Int J Colorectal Dis 25(6):731–740
Norwegian Gastro Intestinal Group (2008) Guidelines for Colorectal and Anal Cancer
Tjandra JJ, Kilkenny JW, Buie WD et al (2005) Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 48(3):411–423
Colorectal Cancer Collaborative Group (2001) Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 358(9290):1291–1304
Wasserberg N, Gutman H (2008) Resection margins in modern rectal cancer surgery. J Surg Oncol 98(8):611–615
Kwok SP, Lau WY, Leung KL, Liew CT, Li AK (1996) Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. Br J Surg 83(7):969–972
Mezhir JJ, Smith KD, Fichera A et al (2005) Presence of distal intramural spread after preoperative combined-modality therapy for adenocarcinoma of the rectum: what is now the appropriate distal resection margin? Surgery 138(4):658–663
Borowski DW, Ratcliffe AA, Bharathan B et al (2008) Involvement of surgical trainees in surgery for colorectal cancer and their effect on outcome. Colorectal Dis 10(8):837–845
Ceelen W, Fierens K, Van NY, Pattyn P (2009) Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer 124(12):2966–2972
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eivind Jullumstrø died of cancer on 20 December 2010. We dedicate this article in thankful memory to him.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Jullumstrø, E., Wibe, A., Lydersen, S. et al. Violation of treatment guidelines — hazard for rectal cancer patients. Int J Colorectal Dis 27, 103–109 (2012). https://doi.org/10.1007/s00384-011-1283-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1283-8