Abstract
Background and aim
Pretherapeutic identification of esophageal squamous cell carcinomas (ESCCs) that are likely to respond to neoadjuvant chemoradiotherapy is important in the attempt to improve the prognosis for patients. In the present study, expression of members of the transforming growth factor-β1 (TGF-β1) signaling pathway was investigated in pretherapeutic biopsies from 97 ESCCs (cT3, cN0/+, cM0) in patients who underwent neoadjuvant chemoradiotherapy (45 Gy plus cisplatin and 5-fluorouracil) and subsequent esophagectomy in the setting of a single-center prospective treatment trial.
Materials and methods
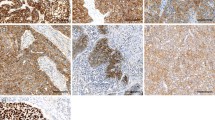
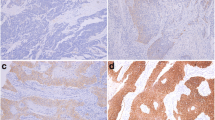
Expression of TGF-β1 and its downstream effectors Smad4 and Smad7 was assessed using quantitative reverse transcription polymerase chain reaction from RNA prepared from pretherapeutic tumor biopsies. The presence of phosphorylated Smad2 was assessed immunohistochemically.
Results
Expression of TGF-β1 (mean 7.8; range 0.0–25.7 arb. units), Smad4 (mean 0.1; range 0.0–0.4 arb. units), and Smad7 (mean 1.6; range 0.4–16.1 arb. units) varied substantially between the patients. Tumors with total or subtotal regression, as determined by histopathological examination after neoadjuvant chemoradiotherapy, showed significantly higher levels of Smad4 mRNA expression than tumors with minor or no regression (P = 0.032). TGF-β1 and Smad7 mRNA expression as well as Smad2 protein expression were of no prognostic value. Expression of the four genes under analysis also showed no impact on the overall survival. In contrast, the overall survival correlated significantly with histopathological regression (P < 0.0001) and to a minor degree also with clinical regression grading (P = 0.0254).
Interpretation
Among the parameters analyzed, only Smad4 was found to have possible predictive value for esophageal squamous cell carcinoma in patients receiving neoadjuvant chemoradiotherapy.

Similar content being viewed by others
References
Levi F, Te VC, Randimbison L, La Vecchia C (2004) Trends in survival for both histologic types of esophageal cancer in Switzerland. Int J Cancer 108:638–639
van Blankenstein M, Looman CW, Hop WC, Bytzer P (2005) The incidence of adenocarcinoma and squamous cell carcinoma of the esophagus: Barrett’s esophagus makes a difference. Am J Gastroenterol 100:766–774
van Meerten E, van der Gaast A (2005) Systemic treatment for oesophageal cancer. Eur J Cancer 41:664–672
Stahl M, Stuschke M, Lehmann N et al (2005) Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23:2310–2317
Stahl M, Wilke H, Stuschke M et al (2005) Clinical response to induction chemotherapy predicts local control and long-term survival in multimodal treatment of patients with locally advanced esophageal cancer. J Cancer Res Clin Oncol 131:67–72
Brücher BLDM, Stein HJ, Zimmermann F et al (2004) Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol 30:963–971
Lordick F, Stein HJ, Peschel C, Siewert JR (2004) Neoadjuvant therapy for oesophagogastric cancer. Br J Surg 91:540–551
Fietkau R (2004) Definitive and neoadjuvant radiochemotherapy of squamous cell carcinoma of the oesophagus. Onkologie 27:39–44
Kawai K, Watabe S, Matsuda M, Sakamoto K, Kamano T (2005) Correlation between expression of orotate phosphoribosyl transferase and 5-fluorouracil sensitivity, as measured by apoptosis index in colorectal cancer tissue. Int J Gastrointest Cancer 35:197–204
Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S (2005) The evaluation of gastric cancer sensitivity to 5-FU/CDDP in terms of induction of apoptosis: time- and p53 expression-dependency of anti-cancer drugs. Oncol Rep 14:609–615
Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S (2005) Apoptosis induced by 5-fluorouracil, cisplatin and paclitaxel are associated with p53 gene status in gastric cancer cell lines. Int J Oncol 26:1563–1567
Zhang QX, Feng R, Zhang W, Ding Y, Yang JY, Liu GH (2005) Role of stress-activated MAP kinase P38 in cisplatin- and DTT-induced apoptosis of the esophageal carcinoma cell line Eca109. World J Gastroenterol 11:4451–4456
Munro AJ, Lain S, Lane DP (2005) P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer 92:434–444
Steele RJ, Lane DP (2005) P53 in cancer: a paradigm for modern management of cancer. Surgeon 3:197–205
Brücher BL, Keller G, Werner M, Müller U, Lassmann S, Cabras AD, Fend F, Busch R, Stein H, Allescher HD, Molls M, Siewert JR, Höfler H, Specht K (2008) Using Q-RT-PCR to measure cyclin D1, TS, TP, DPD, and Her-2/neu as predictors for response, survival, and recurrence in patients with esophageal squamous cell carcinoma following radiochemotherapy. Int J Colorectal Dis 24:69–77
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
Attisano L, Wrana JL (2002) Signal transduction by the TGF-beta superfamily. Science 296:1646–1647
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Stoika R, Yakymovych M, Souchelnytskyi S, Yakymovych I (2003) Potential role of transforming growth factor beta1 in drug resistance of tumor cells. Acta Biochim Pol 50:497–508
Ewan KB, Henshall-Powell RL, Ravani SA et al (2002) Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res 62:5627–5631
Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA (1994) Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 93:892–899
Raynal S, Nocentini S, Croisy A, Lawrence DA, Jullien P (1997) Transforming growth factor-beta1 enhances the lethal effects of DNA-damaging agents in a human lung-cancer cell line. Int J Cancer 72:356–361
Luo K (2004) Ski and SnoN: negative regulators of TGF-beta signaling. Curr Opin Genet Dev 14:65–70
Miyazono K, Maeda S, Imamura T (2004) Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene 23:4232–4237
Landstrom M, Heldin NE, Bu S et al (2000) Smad7 mediates apoptosis induced by transforming growth factor beta in prostatic carcinoma cells. Curr Biol 10:535–538
Edlund S, Bu S, Schuster N et al (2003) Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell 14:529–544
Kim KY, Kim BC, Xu Z, Kim SJ (2004) Mixed lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates transforming growth factor-beta-induced apoptosis in hepatoma cells. J Biol Chem 279:29478–29484
Dai C, Yang J, Liu Y (2003) Transforming growth factor-beta1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem 278:12537–12545
Roberts AB, Wakefield LM (2003) The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A 100:8621–8623
Elliott RL, Blobe GC (2005) Role of transforming growth factor beta in human cancer. J Clin Oncol 23:2078–2093
Fukai Y, Fukuchi M, Masuda N et al (2003) Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer 104:161–166
Zhou Q, Dong Wang L, Du F et al (2002) Changes of TGFbeta1 and TGFbetaRII expression in esophageal precancerous and cancerous lesions: a study of a high-risk population in Henan, northern China. Dis Esophagus 15:74–79
Landis MD, Seachrist DD, Montanez-Wiscovich ME, Danielpour D, Keri RA (2005) Gene expression profiling of cancer progression reveals intrinsic regulation of transforming growth factor-beta signaling in ErbB2/Neu-induced tumors from transgenic mice. Oncogene 24:5173–5190
Fukuchi M, Masuda N, Miyazaki T et al (2002) Decreased Smad4 expression in the transforming growth factor-beta signaling pathway during progression of esophageal squamous cell carcinoma. Cancer 95:737–743
Lazzereschi D, Nardi F, Turco A et al (2005) A complex pattern of mutations and abnormal splicing of Smad4 is present in thyroid tumours. Oncogene 24:5344–5354
Prunier C, Mazars A, Noe V et al (1999) Evidence that Smad2 is a tumor suppressor implicated in the control of cellular invasion. J Biol Chem 274:22919–22922
Natsugoe S, Xiangming C, Matsumoto M et al (2002) Smad4 and transforming growth factor beta1 expression in patients with squamous cell carcinoma of the esophagus. Clin Cancer Res 8:1838–1842
Koliopanos A, Friess H, di Mola FF et al (2002) Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg 26:420–427
Sobin LH, Fleming ID (1997) TNM classification of malignant tumors, fifth edition. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80:1803–1804
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Brücher BLDM, Becker K, Lordick F et al (2006) The clinical impact of histopathological response assessment by residual tumor cell quantification in esophageal squamous cell carcinomas. Cancer 106:2119–2127
Specht K, Richter T, Müller U, Walch A, Höfler MW (2000) Quantitative gene expression analysis in microdissected archival tissue by real-time RT-PCR. J Mol Med 78:B27
Specht K, Richter T, Müller U, Walch A, Werner M, Höfler H (2001) Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol 158:419–429
Rundhaug JE, Park J, Fischer SM (1997) Uncoordinated regulation of mRNA expression of the three isoforms of transforming growth factor-beta in the mouse skin carcinogenesis model. Mol Carcinog 18:115–126
Xie W, Mertens JC, Reiss DJ et al (2002) Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res 62:497–505
Xie W, Rimm DL, Lin Y, Shih WJ, Reiss M (2003) Loss of Smad signaling in human colorectal cancer is associated with advanced disease and poor prognosis. Cancer J 9:302–312
Joshi MB, Shirota Y, Danenberg KD et al (2005) High gene expression of TS1, GSTP1, and ERCC1 are risk factors for survival in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res 11:2215–2221
Mazars A, Lallemand F, Prunier C et al (2001) Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem 276:36797–36803
Osawa H, Nakajima M, Kato H, Fukuchi M, Kuwano H (2004) Prognostic value of the expression of Smad6 and Smad7, as inhibitory Smads of the TGF-beta superfamily, in esophageal squamous cell carcinoma. Anticancer Res 24:3703–3709
Boulay JL, Mild G, Lowy A et al (2003) SMAD7 is a prognostic marker in patients with colorectal cancer. Int J Cancer 104:446–449
Kim YH, Lee HS, Lee HJ et al (2004) Prognostic significance of the expression of Smad4 and Smad7 in human gastric carcinomas. Ann Oncol 15:574–580
Zhang F, Laiho M (2003) On and off: proteasome and TGF-beta signaling. Exp Cell Res 291:275–281
Voorhees PM, Orlowski RZ (2006) The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol 46:189–213
Acknowledgments
We are grateful to Annette Haas and Andrea Harwardt for their skillful assistance. Financial support for this study was received from the Wilhelm Sander Foundation, Grant No. 2005.001.1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pühringer-Oppermann, F., Sarbia, M., Ott, N. et al. The predictive value of genes of the TGF-β1 pathway in multimodally treated squamous cell carcinoma of the esophagus. Int J Colorectal Dis 25, 515–521 (2010). https://doi.org/10.1007/s00384-009-0867-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-009-0867-z