Abstract
Objective
Neoadjuvant chemotherapy in gastric cancer is now standard in the Western world; however, only 30–40% of the patients respond to induction therapy. Pretherapeutic predictors of response and prognosis would be of utmost interest to individualize treatment. Glutathione-S-transferase enzymes detoxify therapeutic drugs such as platin derivates and may influence outcome of the treated patients. Therefore, glutathione-S-transferase (GST) polymorphisms were assessed as predictive markers in cisplatinum-based neoadjuvant-treated gastric cancer.
Materials and methods
DNA was isolated from 139 patients with locally advanced gastric cancer (cT3/4 anyN cM0) before chemotherapy. Multiplex polymerase chain reaction was used for GSTT1 and GSTM1 genes, and allelic discrimination assay with the TaqMan system for the GSTP1 gene.
Results
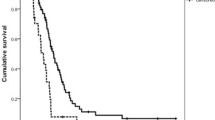
One hundred ten patients could be analyzed for GSTT1 (T-:23; T + 87), 112 for GSTM1 (M-:52; M +:60) and 132 for GSTP1 (Ile/Ile: 55; Ile/Val: 59; Val/Val: 18). There was no significant correlation between any of the GSTT1, GSTM1, or GSTP1 genotypes and patients’ characteristics or histopathological data; only the GSTM1+ genotype was associated with the non-intestinal subtype of the Lauren classification (p = 0.045). GSTT1, GSTM1, and GSTP1 genotypes were not correlated with response to chemotherapy (p = 0.57, p = 0.38, p = 0.33). In R0 resected patients, we found an improved survival for patients with the GSTM1-present genotype compared to patients with the GSTM1-null genotype (p = 0.017). Moreover, the GSTM1-present genotype showed a significantly better tumor-related (p = 0.017) and disease-free survival (p = 0.029).
Conclusion
None of the common GST polymorphisms predicts response in our study, but the GSTM1+ genotype was associated with a better prognosis in completely resected patients. Further investigations on chemotherapy-associated gene polymorphisms are warranted.




Similar content being viewed by others
References
Cunningham D, Allum WH, Stenning SP, Thompson JN et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Boige V, Pignon J, Saint-Aubert B, Lasser P, Conroy T, Bouche O, Segol P, Bedenne L, Rougier P, Ychou M (2007) Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol (Supplement) 25:18S (Abstract)
Ott K, Sendler A, Becker K, Dittler HJ et al (2003) Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer 6:159–167
Lordick F, Stein HJ, Peschel C, Siewert JR (2004) Neoadjuvant therapy for oesophagogastric cancer. Br J Surg 91:540–551
Napieralski R, Ott K, Kremer M, Becker K et al (2007) Methylation of tumor-related genes in neoadjuvant-treated gastric cancer: relation to therapy response and clinicopathologic and molecular features. Clin Cancer Res 13:5095–5102
Ott K, Vogelsang H, Marton N, Becker K et al (2006) The thymidylate synthase tandem repeat promoter polymorphism: a predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int J Cancer 119:2885–2894
Seidegard J, Vorachek WR, Pero RW, Pearson WR (1988) Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A 85:7293–7297
Srivastava SK, Singhal SS, Hu X, Awasthi YC et al (1999) Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys 366:89–94
Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600
Dirven HA, Dictus EL, Broeders NL, van Ommen B et al (1995) The role of human glutathione S-transferase isoenzymes in the formation of glutathione conjugates of the alkylating cytostatic drug thiotepa. Cancer Res 55:1701–1706
Weijl NI, Cleton FJ, Osanto S (1997) Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 23:209–240
La Torre F, Silipigni AM, Orlando A, La T I et al (1997) [Role of free radicals, telomeres, and telomerases in aging and cancerogenesis]. Minerva Med 88:205–214
Cullen KJ, Newkirk KA, Schumaker LM, Aldosari N et al (2003) Glutathione S-transferase pi amplification is associated with cisplatin resistance in head and neck squamous cell carcinoma cell lines and primary tumors. Cancer Res 63:8097–8102
Goekkurt E, Hoehn S, Wolschke C, Wittmer C et al (2006) Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)—novel predictors for response and survival in gastric cancer patients. Br J Cancer 94:281–286
Okuyama T, Maehara Y, Endo K, Baba H et al (1994) Expression of glutathione S-transferase-pi and sensitivity of human gastric cancer cells to cisplatin. Cancer 74:1230–1236
Davies SM, Robison LL, Buckley JD, Tjoa T et al (2001) Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia. J Clin Oncol 19:1279–1287
DeMichele A, Aplenc R, Botbyl J, Colligan T et al (2005) Drug-metabolizing enzyme polymorphisms predict clinical outcome in a node-positive breast cancer cohort. J Clin Oncol 23:5552–5559
Casson AG, Zheng Z, Chiasson D, MacDonald K et al (2003) Associations between genetic polymorphisms of Phase I and II metabolizing enzymes, p53 and susceptibility to esophageal adenocarcinoma. Cancer Detect Prev 27:139–146
Schuhmacher CP, Fink U, Becker K, Busch R et al (2001) Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer 91:918–927
Ott K, Fink U, Becker K, Stahl A et al (2003) Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 21:4604–4610
Menzel C, Dobert N, Rieker O, Kneist W et al (2003) [18F-Deoxyglucose PET for the staging of oesophageal cancer: influence of histopathological subtype and tumour grading]. Nuklearmedizin 42:90–93
Ott K, Dittler HJ, Helmberger H (2000) Preoperative chemotherapy of high dose 5-FU (HDFU) + folinic acid (HDFA) + biweekly Cisplatin without (group A) or with Paclitaxel (group B) in patients with locally advanced adenocarcinomas of the esophagus. Proc Am Soc Clin Oncol 19:287A (Abstract)
Becker K, Mueller JD, Schulmacher C, Ott K et al (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98:1521–1530
Ott K, Weber WA, Lordick F, Becker K et al (2006) Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 24:4692–4698
Ott K, Vogelsang H, Mueller J, Becker K et al (2003) Chromosomal instability rather than p53 mutation is associated with response to neoadjuvant cisplatin-based chemotherapy in gastric carcinoma. Clin Cancer Res 9:2307–2315
Abdel-Rahman SZ, Anwar WA, Abdel-Aal WE, Mostafa HM et al (1998) GSTM1 and GSTT1 genes are potential risk modifiers for bladder cancer. Cancer Detect Prev 22:129–138
Ambrosone CB, Sweeney C, Coles BF, Thompson PA et al (2001) Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res 61:7130–7135
Sweeney C, McClure GY, Fares MY, Stone A et al (2000) Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res 60:5621–5624
Sweeney C, Nazar-Stewart V, Stapleton PL, Eaton DL et al (2003) Glutathione S-transferase M1, T1, and P1 polymorphisms and survival among lung cancer patients. Cancer Epidemiol Biomarkers Prev 12:527–533
Choi SC, Yun KJ, Kim TH, Kim HJ et al (2003) Prognostic potential of glutathione S-transferase M1 and T1 null genotypes for gastric cancer progression. Cancer Lett 195:169–175
Stoehlmacher J, Park DJ, Zhang W, Groshen S et al (2002) Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst 19(94):936–942
Lee JM, Wu MT, Lee YC, Yang SY et al (2005) Association of GSTP1 polymorphism and survival for esophageal cancer. Clin Cancer Res 11:4749–4753
Howells RE, Dhar KK, Hoban PR, Jones PW et al (2004) Association between glutathione-S-transferase GSTP1 genotypes, GSTP1 over-expression, and outcome in epithelial ovarian cancer. Int J Gynecol Cancer 14:242–250
Roder JD, Bottcher K, Siewert JR, Busch R et al (1993) Prognostic factors in gastric carcinoma. Results of the German Gastric Carcinoma Study 1992. Cancer 72:2089–2097
Yang G, Shu XO, Ruan ZX, Cai QY et al (2005) Genetic polymorphisms in glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer 103:52–58
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by KKF 8744168.
Rights and permissions
About this article
Cite this article
Ott, K., Lordick, F., Becker, K. et al. Glutathione-S-transferase P1, T1 and M1 genetic polymorphisms in neoadjuvant-treated locally advanced gastric cancer: GSTM1-present genotype is associated with better prognosis in completely resected patients. Int J Colorectal Dis 23, 773–782 (2008). https://doi.org/10.1007/s00384-008-0490-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-008-0490-4