Abstract
Background and aims
The aim was to determine the toxicity, clinical and immune responses to the murine monoclonal anti-carcinoembryonic antigen (CEA) antibody, PR1A3, in patients with advanced colorectal cancer.
Materials and methods
Fifteen patients with advanced colorectal cancer received either 0.5-, 1.0- or 5.0-mg doses of PR1A3 mixed with 10% w/v Alum adjuvant (Superfos Biosector, Denmark) intradermally at 4-week intervals for 3 months. Patient serum was assessed for anti-idiotypic (Ab2), anti-anti-idiotypic (Ab3) and human anti-mouse antibody (HAMA) reactivity. Peripheral blood mononuclear cell (PBMC) proliferation with phytohaemagglutinin (PHA), CEA and PR1A3, stimulated IL-2, IL-4 and IFN-γ levels and PR1A3-stimulated IL-2 receptor expression during immunotherapy were determined. Comparisons were made with 16 age-matched controls without malignant disease.
Results
Hyperimmune sera from 12 of the 15 patients showed Ab2 reactivity with no detectable Ab3 responses. Strong HAMA reactivity was recorded in 7 of the 15 cases with no adverse clinical effect. Delayed-type hypersensitivity (DTH) responses developed in 12 of the 15 patients. Pre-treatment PBMC proliferation with PHA was subnormal in each patient compared with controls, becoming normal (or supranormal) in all patients during immunisation (P<0.001). PBMC proliferation with CEA and PR1A3 increased during immunotherapy (P<0.001) along with stimulated production of IL-2, IFN-γ and IL-2 receptor expression. Progressive disease was observed in 14 of the 15 patients with minimal toxicity.
Conclusion
PR1A3 generated limited idiotypic responses but robust DTH reactivity in most patients. In vitro PBMC proliferation with mitogens and recall antigens is greatly increased during the course of immunisation, with a shift in stimulated cytokine profile.
Similar content being viewed by others
Introduction
There has been considerable interest in the use of monoclonal antibody-based therapies in colorectal cancer, utilising murine, chimeric and humanised anti-carcinoembryonic antigen (CEA), murine anti-idiotypic and human anti-idiotypic antibodies [1]. Up to 80% of colorectal cancers (CRC) over-express the tumour-associated antigen CEA, with diagnostic and therapeutic monoclonal antibodies (mAb) being well tolerated in patients with advanced disease [2]. Their perioperative adjuvant use in patients with Dukes’ C CRC receiving treatment with the murine mAb 17-1A (anti-Ep-CAM) has shown an early 30% improvement in overall survival and an equivalent reduction in metastatic recurrence with durable survival advantage up to 7 years [3, 4], although preliminary phase III evidence using this antibody in stage III colon cancer has not shown improvement either in disease-free or overall survival [5, 6].
Unmodified mAb therapy in solid epithelial malignancy is thought to function by recruiting several antitumour effector systems, including complement-dependent cell death and antibody-dependent cellular cytotoxicity (ADCC) [7, 8], by the induction of tumour cell apoptosis, cell cycle arrest, abrogation of angiogenesis and by the induction of CEA-specific antibody networks. The latter mechanism was originally predicted by Jerne as a cascading generation of antibodies that specifically target the antigenic epitope of CEA through recognition domains in the antigen-binding site [9]. It is postulated that primary administered murine anti-CEA antibodies (Ab1) will contain components functioning as secondary “epitopes” for recognition by a second line of antibodies referred to as anti-idiotypic antibodies (Ab2) stereochemically resembling CEA itself. These Ab2 antibodies may in certain circumstances be sufficiently immunogenic to stimulate a third tier of recognition anti-anti-idiotypic antibodies (Ab3), which then functionally resemble the administered murine mAb.
Antibody networks, if induced by the primary treatment Ab1, could regulate cancer development by neutralising circulating CEA as well as by binding to surface immunoglobulin receptors on activated immunocytes resulting in tumour-specific T cell receptor stimulation and cytotoxicity. The use of mAb therapy in patients with advanced colorectal cancer has been shown to induce these antibody networks, although the response is dependent upon whether vaccination is by unmodified murine Ab1, murine syngeneic Ab2, or human Ab2 antibody therapy [10–12].
PR1A3 is an IgG1κ mAb that was produced from mice immunised with normal human colonic epithelium, showing binding to upper crypt columnar epithelium and demonstrating high sensitivity and specificity for colorectal tumours of all grades of differentiation [13]. It has been used in the clinic as a sensitive and specific radioimmunoscintigraphic guide for recurrent CEA-bearing colorectal carcinomas [14, 15], showing no effective binding to soluble circulating CEA or related immunoglobulin superfamily group members such as non-specific cross-reacting antigen and biliary glycoprotein [16]. Unlike other CEA antibodies, PR1A3 has a high affinity for its epitope and its recent mapping to the B3 domain of the protein close to the site of membrane attachment but exclusive of the glycosylphosphatidylinositol (GPI) anchor [17] has resulted in the construction of a recombinant recognition protein for PR1A3 (designated NABA), composed of the B3 domain of CEA and domains from BGP and IgG [18]. This has permitted the development of an ELISA that provides avid PR1A3 binding for assays in cell-free systems.
It is anticipated that PR1A3 will function as a valuable tumour targeting agent for clinical use in advanced disease where there are pre-existing high levels of circulating CEA. In this phase I study we evaluated the use of repeated intradermal PR1A3 vaccination in 15 patients with measurable advanced and/or metastatic CRC, assessing their humoral and cell-mediated responses during immunotherapy.
Materials and methods
Patient selection
The study was approved by the local ethics committee (Imperial College School of Medicine and Technology, Hammersmith Hospital) and all patients provided informed consent for treatment and involvement in the project. The study was conducted according to the guidelines for the use of monoclonal antibodies in phase I clinical trials [19]. All patients had CEA-positive, locally advanced, recurrent and/or metastatic CRC that failed to respond to standard therapy (Table 1). Baseline assessment included complete physical examination, chest radiography, thoracoabdominal computerised axial tomography (CT), serum CEA measurement and routine blood chemistries. Patients were eligible for the study if they had measurable disease, an estimated survival expectancy exceeding 4 months and an adequate performance status (ECOG 0-2) on admission to the study. Exclusion criteria were chemotherapy, radiotherapy or steroid treatment within 3 months of commencement of the study, prior PR1A3 radioimmunoscintigraphy, or a history of anaphylaxis or other serious allergy to xenogeneic proteins.
Monoclonal antibody preparation
The production and purification of murine PR1A3 has been previously described [13, 16]. Quality control procedures for monoclonal antibody production met the guidelines set out by the working party on the clinical use of antibodies, where antibody was formulated and packaged into vials of clinical grade by the Hybridoma Development Unit. PR1A3 was mixed with 10% w/v alum precipitate (Alhydrogel; Superfos Biosector, Frederikssund, Denmark) under laminar flow conditions by our local hospital pharmacy for use in three different dosage formulations (0.5, 1 and 5 mg) made up to the same volume (1.4 ml).
Treatment schedule, toxicity and clinical response
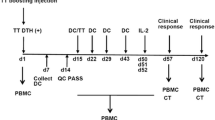
The trial design was a dose escalation phase I study with the primary objective of assessing the toxicity of therapy. Fifteen patients (14 men; mean age 62 years; range 52–80 years) were randomly assigned to the three different doses. Comparisons were made with 16 age-matched controls (11 men; mean age 58 years; range 48–78 years) where the absence of neoplastic disease was established by clinical history, physical examination and routine laboratory testing. Patients were immunised intradermally with the PR1A3-alum gel every 4 weeks for 3 months. Dose escalation was only performed after the lower dose group of five patients had completed their three injections and had reached day 28 with no evidence of dose-limiting toxicity evaluated in accordance with National Cancer Institute criteria and review of blood tests. CT assessment and chest X-ray were performed at the beginning and at the end of immunisation. Blood samples prior to and during treatment were analysed for white cell count, absolute neutrophil count, peripheral blood CD4/CD8 ratios, renal and liver function and serum CEA. Sera were also separated and stored at −70°C until required for serological assays. Clinical responses were defined by the UICC (Union Internationale Contre le Cancer) response criteria for measurable lesions.
Delayed-type hypersensitivity reaction
Delayed-type hypersensitivity (DTH) responsiveness was assessed 48 h after each immunisation. No test dosing methodology was used with the responses being examined on the volar aspect of the non-dominant forearm during the immunisation schedule where induration and erythema was measured as mm2 area using calipers. A full-thickness 2-mm punch biopsy (Stiefel, Offenbach, Germany) of immunisation sites was taken in six patients under local anaesthesia for immunohistochemistry at the end of the vaccination schedule. Samples were orientated on cork disks for immediate freezing in isopentane and storage at −80°C and for fixation and paraffin embedding. Fixed specimens were stained with haematoxylin and eosin and frozen samples were evaluated for dendritic cells (with the mouse monoclonal antibodies RFD1/Factor XIIIa and CD1a; NA1/34 Clone; DAKO, Glostrup, Denmark) in accordance with previously described techniques [20], CD4, CD8 and CD 22 antibodies (Southern Biotechnology Associates, Birmingham, AL, USA), and with the pan-macrophage marker EBM 11 (anti-CD68; DAKO, Glostrup, Denmark) [21]. Appropriate positive and negative antibody controls were used and comparisons were made with samples of normal skin and purified protein derivative (PPD)-induced DTH-positive controls available from a skin tissue bank (Royal London Hospitals NHS Trust Skin tumour Laboratory).
Human anti-mouse antibodies
Levels of human anti-mouse antibodies (HAMA) were determined in patient sera before and after the vaccination schedule using a commercial HAMA ELISA (Immunomedics, Morris Plains, NJ, USA) following the manufacturer’s instructions with values reported from a standard dilution curve as nanograms of precipitable antibody equivalents per ml against a known reference standard of anti-mouse IgG containing 220 ng equivalents/ml [22].
Ab2 and Ab3 assays
Anti-idiotypic (Ab2) antibodies were detected by ELISA using 96-well microtitre plates coated with purified F(ab) fragments of PR1A3 (20 μg/ml). F(ab) fragments were generated by digestion of PR1A3 (4 mg/ml in 0.1 M sodium acetate, pH 6.5, 50 mM dithiothreitol) with papain (0.2 mg/ml) for 4 h at 37°C and purified by Superose and MonoQ chromatography. Serum reactivity was detected using an alkaline phosphatase-conjugated goat anti-human Fc antibody (Sigma, Poole, UK) and developed with p-nitrophenyl phosphate substrate (Sigma). Plates were read on a microplate autoreader (Labsystems Multiskan, Basingstoke, UK) at 405 nm. Positive tests were reported if maximal serum concentrations had optical density (OD) values >2 SD beyond a panel of control sera derived from age-matched patients without malignant disease or murine antibody exposure (n=16). Patients were also screened for Ab3 levels by ELISA where 96-well microtitre plates were coated with a purified recombinant hybrid antigen (NABA) containing the CEA B3 domain, which has previously been shown to contain the PR1A3 epitope [17]. Antibody binding was determined using alkaline phosphatase-conjugated goat anti-human antibody and developed as described above.
Peripheral blood mononuclear cell proliferation assay
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinised whole blood by Ficoll-Hypaque (Nycomed, Asker, Norway) density gradient centrifugation. PBMCs were resuspended in complete RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% pooled normal human AB serum, 2 mM l-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. All proliferation assays were performed using fresh PBMCs. Cells were added to 96-well flat-bottomed plates (Pierce-Warriner, Chester, UK) at 1×106 cells/well and stimulated with purified CEA, PR1A3, or control antibody, HMFG1 (0.01–10 μg/ml) or phytohaemagglutinin (PHA; 0.1–10 μg/ml; Sigma). Incubation doses of antibodies and CEA were based on studies of a similar nature during mAb immunotherapy with other murine Ab1 antibodies (GA 733-2 and 3 H1) directed against CEA [11]. Cells were cultured for 3 days and pulsed with 1 μCi/well of tritiated thymidine (Amersham, Amersham, UK) for 18 h, harvested, and radioactivity was incorporated into DNA estimated by scintillation counting (Wallac, Milton Keynes, UK). The stimulation index (SI) was calculated as mean cpm of stimulated wells/mean cpm of control wells (supplemented medium only).
Immunofluorescent labelling and flow cytometry
Peripheral blood mononuclear cells (1×106 cells) were cultured in 96-well plates at 37°C for 7 days in the absence or presence of PR1A3 or control antibody (10 μg/ml) for determination of CD25-expressing CD4+ PBMCs as reported by Kosmas et al. [23]. Cells were harvested and labelled with phycoeryrthritin (PE)-conjugated mouse anti-human-CD4, fluorescein isothiocyanate (FITC)-conjugated mouse anti-human-CD25 (Pharmingen, Oxford, UK) or isotype-matched control antibodies (Caltag, Towcester, UK). Antibody labelling was assessed by flow cytometry with a FACScan (Becton Dickinson, Oxford, UK) where gates were set on viable cells according to forward and side scatter and data were analysed using the Cell Quest software package.
Cytokine assays
Patient PBMCs (1×107 cells) were cultured with PHA (10 μg/ml) in 96-well microtitre plates and cell-free supernatant was harvested for IL-2 analysis after 48 h incubation, following preliminary kinetics experiments of control lymphocytes derived from age-matched volunteers without malignant disease (data not shown).
As initial experiments failed to show detectable levels of IL-4 in the supernatants of PHA-stimulated PBMCs, IL-4 production was detected following PBMC stimulation for 12 h using a combination of 10 ng/ml phorbol-12-myristate 13-acetate (PMA; Sigma) and 10 μmol/ml calcium ionomycin (Calbiochem, Nottingham, UK) as described previously [24]. IL-2 levels were quantitated by bioassay using the IL-2-dependent mouse cytotoxic lymphocyte cell line CTLL-2 [25]. IL-4 levels were quantified by ELISA using the anti-human IL-4 antibody (426-1A6-10; NIBSC, Potters Bar, UK) at 5 μg/ml for coating and the biotinylated antibody 426-8D4-8 (Pharmingen) for detection as previously described [26] IFN-γ levels were also measured in an ELISA using commercially available antibodies where the sensitivity for both IL-4 and IFN-γ ELISAs was 20 pg/ml. In all cytokine assays, WHO International standards or reference agents available from NIBSC were used.
Statistics
For variables that were measured only once for each subject, unpaired t tests or regression analyses were employed. For measurements taken at different immunisation times and under varying doses of mitogen/antigen exposure, random effects regression models were used. The normality of residuals for all data was assessed by the Shapiro–Francia W′ test. Bartlett’s test was employed to ensure equal variances in factors and where these assumptions were not met, data were transformed. Spearman’s rank correlation was used to determine the relationship between cytokines produced and IL-2 receptor expression. All P values <0.05 are reported.
Results
Clinical response and toxicity
The immunological and clinical responses of the patients are shown in Tables 1 and 2. No serious toxicity was observed in any of the patients with only local reactions (erythema and induration) noted at the injection sites. Three patients (patients 6, 7 and 12) experienced mild fever and flu-like symptoms lasting 48 h after their final injection. None of the patients had observable lymphadenopathy and no haematological (total white blood cell and absolute neutrophil counts), hepatic or renal toxicity was observed, nor was there any observable change in peripheral CD4/CD8 ratios (data not shown). All but one patient (patient 8) eventually developed progressive disease with serial monitoring of serum CEA correlating with disease progression in all cases (data not shown).
DTH response to PR1A3
Positive DTH responsiveness was recorded when at least one dimension of skin induration or observable erythema exceeded 10 mm in diameter (Fig. 1). During the course of PR1A3 vaccination, 12 of the 15 patients developed DTH responses, although 3 patients had reduced responses following their third immunisation compared with their second (patients 1, 4 and 14). The mean area of induration measured after the first immunisation was 86.8±39.07 mm2 (range 2–459 mm2), 769±246.9 mm2 (range 1–3,600 mm2) after the second immunisation and 618.4±199.8 mm2 (range 0–2,000 mm2) after the third immunisation. Haematoxylin and eosin staining of immunisation site biopsies confirmed typical type IV hypersensitivity responses in the six biopsied cases, with extensive perivascular mononuclear cell infiltration of the dermis and dermal oedema. CD1a, RFD1 and EBM11 immunohistochemistry showed diffuse dermal infiltrates of dendritic antigen-presenting cells (Fig. 2) and macrophages, with only scant CD4, CD8 and CD22 infiltration.
HAMA reactivity
When sera was assessed for HAMA responses during the course of PR1A3 immunisations, patients fell into two broad categories of high responders (n=7; post-treatment HAMA reactivity 871.3±39.1 ng/ml, range 720.2–1,011.4 ng/ml) and non/low responders (n=8; post-treatment HAMA reactivity 88.4±25.7 ng/ml, range 7.6–211.7 ng/ml; Fig. 3). None of the patients showed a positive pre-treatment HAMA reaction.
Ab2 and Ab3 production following immunisation with PR1A3
Ab2 antibodies specific to the F(ab) fragment of PR1A3 progressively developed in 12 of the 15 patients following intradermal immunisation. An example of one of the progressive ELISAs is shown in Fig. 4 (patient 5). Two patients (patients 1 and 6) showed pre-existing low-level Ab2. Mixed model ANOVA for repeated measurements from each subject failed to show an effect of the PR1A3 immunising dose on Ab2 production (P=0.29). No evidence of Ab3 antibody production was observed in any of the PR1A3-treated patient sera using the NABA (PR1A3 epitope-bearing) ELISA.
PBMC responses to mitogen, antibody and CEA following vaccination
Regression analyses based on the in vitro dose of mitogen used demonstrated that all patients tested (n=12) had subnormal dose-dependent responses to PHA prior to PR1A3 immunisation when compared with age-matched controls without malignant disease (P<0.001). PHA responsiveness was significantly upregulated in all patients during immunisation (P<0.001) with no significant differences noted in responses by the PBMCs of the treated groups between the first or second immunisations (P=0.17; Fig. 5; Table 2).
Stimulation indices for peripheral blood mononuclear cells (PBMCs) in vitro during PR1A3 immunotherapy. a Phytohaemagglutinin (PHA), b Carcinoembryonic antigen (CEA), c PR1A3, d HMFG-1. Doses of mitogen/antigens are in μg/ml. NC normal controls, PRE pre-immunisation, POST 1st first immunisation, POST 2nd second immunisation. Doses of mitogen/antigen are in μg/ml.
Following immunisation with PR1A3, patient PBMCs proliferated when incubated with both PR1A3 (pre- vs. post-immunisation P<0.001; first vs. second immunisation P<0.001) and CEA (pre- vs. post-immunisation P<0.001; first vs. second immunisation P=0.04), with maximal responses occurring after the second injection, although the stimulation indices did not approach the values obtained with PHA. These effects were not PR1A3 dose-dependent on analysis and during immunisation there was minimal proliferation observed in co-culture with the idiotypically irrelevant IgG 1 mAb, HMFG1.
Induction of CD25 expression in vitro
Patient PBMCs incubated with PR1A3 showed a significant increase in the proportion of CD25+/CD4+ cells during the course of immunisation compared with PBMCs incubated with enriched medium only or with HMFG1. These values with PR1A3 increased from 4.6±1.1% before immunisation to 10.5±1.8% following the first immunisation, 16.4±3.8% after the second immunisation and 26.2±3.5% by the final vaccination (P<0.001). Percentage values for double-staining CD4+ PBMCs on incubation with HMFG-1 were 4.1±0.9% prior to immunisation and 4.4±0.6, 4.5±0.5 and 9.2±2.1% respectively after each PR1A3 immunisation (Fig. 6).
Cytokine production during PBMC stimulation in vitro
Prior to PR1A3 immunisation, none of the patients produced IL-2 following PHA stimulation. During PR1A3 vaccination, significant IL-2 upregulation was observed (post first immunisation 3.83±1.6 U/ml; post second immunisation 11.5±3.3 U/ml and post third immunisation 12.8±4.4 U/ml; P<0.001). Following completion of PR1A3 treatment, stimulated IL-2 levels from PBMCs exceeded IL-2 production by PBMCs derived from control subjects (control PBMC IL-2=3.83±1.32 U/ml; P<0.001; Fig. 7). IFN-γ production in response to PHA stimulation was significantly lower in the pre-treatment patient group compared with control PBMCs (309±78 vs. 4,737.5±845.2 pg/ml respectively; P<0.001). Despite IFN-γ levels progressively increasing during PR1A3 immunotherapy (P<0.001), IFN-γ production remained subnormal compared with controls during the immunisation schedule (1,800±820 pg/ml after the first immunisation and 2,895±992 pg/ml after the second immunisation; insufficient samples for comparison after the third immunisation). IL-4 levels from pre-PR1A3 treatment patient PBMCs were found to be significantly higher compared with control PBMCs (mean control IL-4=919.4±163.9 pg/ml vs. mean pre-treatment IL-4=1,400.6±265.7; P<0.001) and there was a significant overall reduction in IL-4 production during the course of PR1A3 immunisation (910.8+ 370.6 pg/ml after the first immunisation and 606.3+258.9 pg/ml after the second immunisation: P<0.001; insufficient samples for comparison after the third immunisation).
Supernatant cytokine production by PBMCs in vitro during PR1A3 immunisation (see Materials and methods). a IL-2 was measured by bioassay with CTLL-2 cells and b IL-4. c IFN-γ was measured by ELISA.
There was sporadic low level IFN-γ production at variable times in the immunisation schedule in the case of CEA (patient 1, 20 pg/ml after two immunisations; patient 4, 175 pg/ml after three immunisations and patient 14, 150 pg/ml after one immunisation) and in the case of PR1A3 after the second immunisation (patient 4, 175 pg/ml). No correlation was evident between cytokine levels at any time point during immunisation or between stimulated IL-2 levels and the percentage of IL-2 receptor-expressing CD4+ lymphocytes during the course of immunotherapy.
Discussion
The PR1A3 antibody is in theory, an effective reagent for the targeting of CRC as it binds preferentially to cell-bound and not to soluble CEA; a potential advantage in metastatic or locally advanced disease where there are frequently high levels of circulating tumour-associated antigen capable of complexing with other anti-CEA antibodies. In the present study there was no evidence of treatment toxicity, similar to other studies in CRC using different mAbs [3, 5]. Progression of measurable disease was, however, observed in nearly all patients, a finding that has been recently reported in phase II trials of patients with metastatic CRC using the human anti-idiotypic antibody 105AD7 [27]. Immune responses resulted during the treatment schedule in most patients as evidenced by DTH reactivity, immunisation site dendritic cell and macrophage infiltration and by Ab2 production, although none of the patients showed Ab3 reactivity. It is accepted that some of this DTH responsiveness may have been due to anti-murine reactivity, although our group felt that it was unethical to subject these patients to contralateral exposure to an isotypically identical but idiotypically irrelevant murine IgG1 monoclonal. There was high HAMA reactivity detected in half of the treated patients, although without attendant morbidity. Subnormal pre-treatment in vitro PHA responsiveness by PBMCs derived from our patients was normalised in all cases during the course of immunotherapy with an antigen-specific increase in PBMC proliferation in vitro with repeated exposure to CEA and PR1A3. This was accompanied by an increase in PBMC IL-2 and IFN-γ production, a reduction in IL-4 generation and an antigen-specific increase in IL-2 receptor (CD25)-expressing CD4+ PBMCs on co-culture with the immunising antibody.
Only half of the patients developed high HAMA titres during the course of the study. In theory, the presence of HAMA may neutralise mAb directly by immune complex formation, leading to rapid antibody clearance and even serious hypersensitivity, although studies have failed to show any correlation between tumour relapse and HAMA levels in patients with minimal residual CRC treated with adjuvant Ab1 therapy [28]. Over half the patients in the study developed Ab2 antibodies with two patients showing pre-existing Ab2 reactivity; the latter finding having previously been reported in patients with a range of solid tumours undergoing murine Ab1 therapy [29, 30]. We were unable, however, to show any Ab3 reactivity, a finding that has been reported before in advanced CRC using human Ab2 immunotherapy [31]. Other studies, have, however, correlated Ab3 reactivity with overall cancer-specific outcome during therapy [10], although detection of Ab3 species may be more dependent upon performance status of the patients undergoing treatment, where only fitter patients living longer may be able to generate extended antibody networks. There is also the potential that Ab2/Ab3 complexes may develop during therapy (affecting idiotope recognition by ELISA) [32], or that metastatic deposits will function as an “antibody sink” for circulating Ab3, limiting their detection in sera as disease progresses.
There is substantial evidence to show that patients with advanced malignancy have global impairment in their cell-mediated immune responses, which in some cases correlates with disease stage and clinical course. These changes have been manifest as alterations in DTH reactivity to recall antigens, diminished lymphocyte proliferation in vitro to mitogenic stimuli and alterations in T-cell receptor signalling of both PBMCs and tumour-infiltrating lymphocytes [33]. In this study, not only was there upregulation of PHA responsiveness by PBMCs in vitro but also both soluble CEA and the immunising Ab1 functioned as recall antigens, a finding that mirrors that of serum cytokine changes recently noted in patients with advanced colorectal cancer treated with combination chemoimmunotherapy [34]. The effects on PBMC reactivity in our study were not dose-dependent; in some patients, stimulation indices with very small concentrations of CEA approached pre-treatment responses to PHA. This type of finding has been noted before by Kosmas et al. in patients with advanced ovarian cancer undergoing intraperitoneal mAb therapy with radiolabelled and chelate-conjugated HMFG1 [23], as well as in patients with CRC treated with murine Ab2 [35], although stimulation indices achieved in our study were much higher than in these other reports. Our findings were accompanied by only poor responsiveness in vitro by PBMCs to an idiotypically irrelevant murine monoclonal IgG1 (HMFG1), supporting the view that antigen-specific immunotherapy has an important bearing on lymphocyte recognition and memory in CRC. In this respect, Lanzavecchia et al. [36] have shown that T cell clones can be raised during mAb administration that are specific for murine immunoglobulin, but which are also capable of killing target tumour cells bound to mAb recognising tumour-associated antigen.
The separation by Mosmann et al. [37] of murine CD4+ PBMCs based on cytokine profiles into Th1 cells capable of producing IL-2, IFN-γ and TNF-β and Th2 cells that secrete IL-4, IL-5, IL-6, IL-10 and IL-13 has implications for the classification of human PBMCs. It is suggested that Th1 dominance is important for the development of DTH responsiveness and activation of cytotoxic lymphocyte function, and that Th2 dominance is more representative of humoral responsiveness. Pellegrini et al. [38] have shown a predominance of Th2 PBMCs from patients with CRC that is stage-dependent and Tsitsilonis and colleagues [39] have recently demonstrated an enhancement of serum Th1 cytokine production during mAb therapy (17-1A) at different stages of CRC. Our study expands on these findings by showing stimulated PBMC conversion in CRC from a Th2-dominant profile to a Th1-dominant phenotype during highly specific antigen-specific immunotherapy. Although both favourable and unfavourable T-helper cell responses can occur alongside progressing tumour burdens [40], more research is needed concerning the importance of the Th1/Th2 paradigm in CRC to define whether it may be predictive either of chemotherapeutic or immunotherapeutic response. Antibody-dependent cellular cytotoxicity (ADCC) appears to be a principal immune effector mechanism for tumour cell killing during unconjugated monoclonal antibody therapy, perhaps stimulating apoptosis and cell cycle arrest as well as inhibiting the process of angiogenesis necessary for metastatic spread [41]. Each is an effect that may be dependent upon the inherent cytokine milieu as well as being enhanced by the concomitant use of colony-stimulating factors [42, 43] The future direction of our work needs to assess the effect this novel antibody has on in vitro ADCC activity.
In conclusion, this antibody proved safe for repeated intradermal use, eliciting antigen-specific humoral and cell-mediated responses in vitro and converting PBMC cytokine production profiles to a pattern more conducive to tumour cytotoxicity. This approach has recently been adopted by Tsitsilonis et al. [39] using the 17-1A monoclonal antibody Edrecolomab, where this group showed enhanced PBMC proliferative capacity and increased lymphokine-activated killer activity against tumour-sensitive targets as well as changes in circulating serum cytokine levels to a more immuno-enhancing pattern. PR1A3 mAb therapy is a treatment of low toxicity and it is likely to be more appropriate for use in patients with minimal residual disease where there is less attendant immunosuppression. The results of this study justify exploration of its clinical use in a minimal residual disease setting.
References
Mellstedt H (2003) Monoclonal antibodies in human cancer. Drugs Today (Barc) 39 [Suppl]:C1–C16
Mellstedt H, Frodin J-E, Masucci G, Ragnhammar, Fagerberg J, Hjelm AL, Shetye J, Wersall P, Osterborg A (1991) The therapeutic use of monoclonal antibodies in colorectal carcinoma. Semin Oncol 18:462–477
Riethmuller G, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmaier H, Hirsch R, Pichlmayr R et al (1994) Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes’ C colorectal carcinoma. Lancet 343:1177–1183
Riethmuller G, Holz E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Funke I, Pichlmaier H, Hirche H, Buggisch P, Witte J, Pichlmayr R (1998) Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven year outcome of a multicenter randomized trial. J Clin Oncol 16:1788–1794
Punt CJA, Nagy A, Douillard JY et al (2001) Edrecolomab (17-1A antibody) alone or in combination with 5-fluorouracil based chemotherapy in the adjuvant treatment of stage III colon cancer: results of a phase III study. Proc Am Soc Clin Oncol 20:123(A)
Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, Barone C, Fountzilas G, Riess H, Moylan E, Jones E, Dethling J, Colman J, Coward L, MacGregor S (2002) Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet 360:671–677
Herlyn D, Koprowski H (1981) Monoclonal anticolon carcinoma antibodies in complement-dependent cytotoxicity. Int J Cancer 27:769–774
Adams DO, Hall T, Steplewski Z, Koprowski H (1984) Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype containing increasing numbers of macrophages activated for distinctive form of antibody dependent cytolysis. Proc Natl Acad Sci USA 81:3506–3510
Jerne NK (1974) Towards a network theory of the Immune system. Ann Immunol (Paris) 125C:373–389
Fagerberg J, Frodin J-E, Wigzell H, Mellstedt H (1993) Induction of an immune network cascade in cancer patients treated with monoclonal antibodies (ab1). I. May induction of ab1-reactive T cells and anti-anti-idiotypic antibodies (ab3) lead to tumor regression after mAb therapy? Cancer Immunol Immunother 37:264–270
Foon KA, Chakraborty M, John WJ, Sherratt A, Kohler H, Bhattacharya-Chatterjee M (1995) Immune response to the carcinoembryonic antigen in patients with an anti-idiotype antibody vaccine. J Clin Invest 96:334–352
Durrant LG, Denton GWL, Jacobs E, Mee M, Moss R, Austin EB, Baldwin RW, Hardcastle JD, Robins RA (1992) An idiotypic replica of carcinoembryonic antigen inducing cellular and humoral responses directed against human colorectal tumors. Int J Cancer 50:811–816
Richman PI, Bodmer WF (1987) Monoclonal antibodies to human colorectal epithelium: markers for differentiation and tumor characterization. Int J Cancer 39:317–328
Lunniss PJ, Skinner S, Britton KE, Granowska M, Morris G, Northover JM (1999) Effect of radioimmunoscintigraphy on the management of recurrent colorectal cancer. Br J Surg 86:244–249
Kim JC, Kim WS, Ryu JS, Oh SJ, Lee DH, Koo KH, Roh SA, Kim HC, Yu CS, Kang GH, Bodmer WF (2000) Applicability of CEA-specific monoclonal antibodies to radioimmunoguided surgery for human colorectal carcinoma. Cancer Res 60:4825–4829
Granowska M, Jass JR, Britton KE, Northover JM (1989) A prospective study of the use of 111In-labelled monoclonal antibody against CEA in colorectal cancer and of some biological factors affecting its uptake. Int J Colorect Dis 4:97–108
Durbin H, Young S, Stewart LM, Wrba F, Rowan AJ, Snary D, Bodmer WF (1994) An epitope on carcinoembryonic antigen defined by the clinically relevant antibody PR1A3. Proc Natl Acad Sci USA 91:4313–4317
Stewart LM, Young S, Watson G, Mather SJ, Bates PA, Band HA, Wilkinson RW, Ross EL, Snary D (1999) Humanisation and characterization of PR1A3, a monoclonal antibody specific for cell-bound carcinoembryonic antigen. Cancer Immunol Immunother 47:299–306
Begent RHJ, Searle F, Keep PA et al (1986) Operation manual for control, production, preclinical toxicity and phase I trials of anti-tumour antibodies and drug-antibody conjugates. Joint Committee Cancer Research Campaign. Br J Cancer 54:557–568
Bos JD, van Garderen ID, Krieg SR, Poulter LW (1986) Different in situ distribution patterns of dendritic cells having Langerhans (t6+) and interdigitating (RFD1+) cell immunophenotype in psoriasis, atopic dermatitis and other inflammatory dermatoses. J Invest Dermatol 87:358–361
Betjes MG, Haks MC, Tuk CW, Beelen RH (1991) Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology 183:79–87
LaFontaine GS, Hansen HJ, Weiss BF et al (1988) Enzyme immunoassay for the detection of circulating immunoglobulins in humans to mouse monoclonal antibody (HAMA). 3rd International Conference of Monoclonal Antibody Immunoconjugates for Cancer, San Diego, 4–6 February 1998. Abstract 77
Kosmas C, Epenetos AA, Courtenay-Luck NS (1991) Patients receiving murine monoclonal antibody therapy for malignancy develop T cells that proliferate in vitro in response to these antibodies as antigens. Br J Cancer 73:494–500
Neuhaus TJ, Wadhwa M, Callard R, Barratt TM (1995) Increased IL-2, IL-4 and interferon-gamma (IFN-γ) in steroid-sensitive nephrotic syndrome. Clin Exp Immunol 100:475–479
Wadhwa M, Bird C, Dilger P et al (2000) Quantitative biological assays for cytokines. In: Balkwill FR (ed) Cytokine cell biology: a practical approach, 3rd edn. Oxford University Press, Oxford, pp 207–239
Bird C, Wadhwa M, Thorpe R (1991) Development of immunoassays for human IL-3 and IL-4, some of which discriminate between different recombinant DNA-derived molecules. Cytokine 3:562–567
Maxwell-Armstrong CA, Durrant LG, Buckley TJ, Scholefield JH, Robins RA, Fielding K, Monson JR, Guillou PJ, Calvert H, Carmichael J, Hardcastle JD (2001) Randomized double-blind phase II survival study comparing immunization with the anti-idiotypic monoclonal antibody 105AD7 against placebo in advanced colorectal cancer. Br J Cancer 84:1441–1446
Gruber R, van Haarlem LJM, Warnaar SO, Holz E, Riethmuller G (2000) The human antimouse immunoglobulin response and the anti-idiotypic network have no influence on outcome in patients with minimal residual colorectal cancer treated with monoclonal antibody 17-1A. Cancer Res 60:1921–1926
Herlyn D, Wettendorff M, Schmoll E, Iliopoulos D, Schedel I, Dreikhausen U, Raab R, Ross AH, Jaksche H, Scriba M et al (1987) Anti-idiotype immunization of cancer patients: modulation of the immune response. Proc Natl Acad Sci USA 84:8055–8059
Kirman I, Jenkins D, Fowler R, Whelan RL (2003) Naturally occurring antibodies to epithelial cell adhesion molecule (EpCAM). Dig Dis Sci 48:2306–2309
Durrant LG, Buckley DJ, Spendlove I, Robins RA (1997) Low doses of 105AD7 cancer vaccine preferentially stimulate anti-tumour T-cell immunity. Hybridoma 16:23–26
Friguet B, Chaffotte A, Djavadi-Ohanianace L, Godberg ME (1985) Measurements of the true affinity constant in solution of antigen–antibody complexes by ELISA. J Immunol Methods 77:305
Reichert TE, Rabinowich H, Johnson JT, Whiteside TL (1998) Immune cells in the tumor microenvironment: mechanisms responsible for signaling and functional defects. J Immunother 21:295–308
Hjelm Skog AL, Wadhwa M, Hassan M, Gharizadeh B, Bird C, Ragnhammar P, Thorpe R, Mellstedt H (2001) Alteration of interleukin 2 (IL-2) pharmacokinetics and function by IL-2 antibodies induced after treatment of colorectal carcinoma patients with a combination of monoclonal antibody, 17-1A, granulocyte macrophage colony-stimulating factor and IL-2. Clin Cancer Res 7:1163–1170
Pervin S, Sherratt A, Wang H-T et al (1996) Proliferation of T cells from colon cancer patients with peptides based on the structure of an anti-idiotype antibody mimicking CEA. Am Assoc Cancer Res 37:473–474 (A3231)
Lanzavecchia A, Abrignani S, Scheidegger D, Obrist R, Dorken B, Moldenhauer G (1988) Antibodies as antigens: the use of mouse monoclonal antibodies to focus human T cells against selected targets. J Exp Med 167:345–352
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clones. I. Definition according to lymphokine activities and secreted proteins. J Immunol 136:2348–2357
Pellegrini P, Berghella A-M, Del Beato T, Cicia S, Adorno D, Casciani CU (1996) Dysregualtion in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother 42:1–8
Tsitsilonis OE, Tsavaris NB, Kosmas C, Gouveris P, Papalambros E (2003) Immune changes in patients with colorectal cancer treated by adjuvant therapy with monoclonal antibody 17-1A: a pilot study. J Chemother 15:387–393
Ghosh P, Komschlies KL, Cippitelli M, Longo DL, Subleski J, Ye J, Sica A, Young HA, Wiltrout RH, Ochoa AC (1995) Gradual loss of T-helper 1 populations in spleen of mice during progressive tumor growth. J Natl Cancer Inst 87:1478–1483
Takamaku K, Baba K, Arinaga S, Li J, Mori M, Akiyoshi T (1996) Apoptosis in antibody-dependent monocyte-mediated cytotoxicity with monoclonal antibody 17-1A against human colorectal carcinoma cells: enhancement with interferon gamma. Cancer Immunol Immunother 43:220–225
Ragnhammar P, Fagerberg J, Frodin JE, Hjelm AL, Lindemalm C, Magnusson I, Masucci G, Mellstedt H (1993) Effect of monoclonal antibody 17-1A and GM-CSF in patients with advanced colorectal carcinoma—long-lasting, complete remissions can be induced. Int J Cancer 53:751–758
Flieger D, Spengler U, Beier I, Kleinschmidt R, Hoff A, Varvenne M, Sauerbruch T, Schmidt-Wolf I (1999) Enhancement of antibody dependent cellular cytotoxicity (ADCC) by combination of cytokines. Hybridoma 18:63–68
Acknowledgements
We wish to thank David Ellison, Heather Band and Gillian Lewis for antibody supply, Amy Hider and Paul Bassett for statistical advice, Helen Quinn for patient recruitment and Nick Tidman for provision of control skin samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zbar, A.P., Thomas, H., Wilkinson, R.W. et al. Immune responses in advanced colorectal cancer following repeated intradermal vaccination with the anti-CEA murine monoclonal antibody, PR1A3: results of a phase I study. Int J Colorectal Dis 20, 403–414 (2005). https://doi.org/10.1007/s00384-004-0726-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-004-0726-x