Abstract
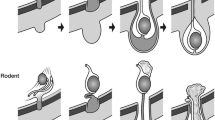
Recent studies of testicular descent suggest not only that the gubernaculum does not initially attach to the scrotum, but also that it must migrate from the groin. Two findings suggest that the gubernaculum may behave like an embryonic limb bud during this phase. First, the active growth centre is at the distal tip of the gubernaculum. Secondly, the gubernaculum is loose in the subcutaneous tissues beneath Scarpa's fascia. The free protrusion of the gubernaculum from the abdominal wall was so reminiscent of a developing embryonic limb bud, we thought that the biological controls of both processus may be similar. This review examines what is known about vertebrate limb bud development, and compares the mechanisms to what has recently been discovered in the gubernaculum. The hypothesis that both processes may be similar is initially consistent with the current facts, encouraging us to investigate this further experimentally.





Similar content being viewed by others
References
Hrabovszky Z, Pilla ND, Yap T, Farmer PJ, Hutson JM, Carlin JB (2002) Role of the gubernacular bulb in cremaster muscle development of the rat. Anat Record 267:159–165
Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA et al (2001) Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol 236(2):421–435
Mic FA, Haselbeck RJ, Cuenca AE, Duester G (2002) Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129(9):2271–2282
Gibson-Brown JJ, Agulnik SI, Silver LM, Niswander L, Papaionnou VE (1998) Involvement of T-box genes Tbx2–Tbx5 in vertebrate limb specification and development. Development 125:2499–2509
Saito D, Yonei-Tamura S, Kano K, Ide H, Tamura K (2002) Specification and determination of limb deformity: evidence for inhibitory regulation of Tbx gene expression. Development 129:211–220
Mariani FV, Martin GR (2003) Deciphering skeletal patterning: clues from the limb. Nature 423(6937):319–325
Dudley AT, Ros MA, Tabin CJ (2002) A re-examination of proximodistal patterning during vertebrate limb development. Nature 418:539–544
Nelson CE, Morgan BA, Burke AC, Laufer E, DiMambro E, Murtaugh LC et al (1996) Analysis of Hox gene expression in the chick limb bud. Development 122:1449–1466
Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T et al (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21(1):138–141
Ohuchi H, Nakagawa T, Itoh N, Noji S (1999) FGF10 can induce Fgf8 expression concomitantly with En1 and R-fng expression in chick limb ectoderm, independent of its dorsoventral specification. Dev Growth Differ 41(6):665–673
Sun X, Mariani FV, Martin GR (2002) Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418(6897):501–508
Boulet AM, Moon AM, Arenkiel BR, Capecchi MR (2004) The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol 273(2):361–372
Tickle C (2002) Molecular basis of vertebrate limb patterning. Am J Med Genet 112(3):250–255
Stratford T, Horton C, Maden M (1996) Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr Biol 6(9):1124–1133
Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C et al (2000) A model for anteroposterior patterning of the vertebrate limb based on sequential long-and short-range Shh signalling and Bmp signalling. Development 127:1337–1348
Zakany J, Kmita M, Duboule D (2004) A dual role for Hox genes in limb anterior–posterior asymmetry. Science 304(5677):1669–72
Deschamps J (2004) Developmental biology. Hox genes in the limb: a play in two acts. Science 304(5677):1610–1611
Chen H, Johnson RL (2002) Interactions between dorsal–ventral patterning genes lmx1b, engrailed-1 and wnt-7a in the vertebrate limb. Int J Dev Biol 46(7):937–941
Pizette S, Abate-Shen C, Niswander L (2001) BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development 128(22):4463–4474
Parr BA, McMahon AP (1995) Dorsalizing signal Wnt-7a required for normal polarity of D–V and A–P axes of mouse limb. Nature 374(6520):350–353
Hutson JM, Hasthorpe S (2005) Testicular descent and cryptorchidism: the state of the art in 2004. J Pediatr Surg 40(2):297–302
Heyns CF, Hutson JM (1995) Pediatric urology: review article: historical review of theories on testicular descent. J Urol 153(3):754–767
Haack H, Gruss P (1993) The establishment of murine Hox-1 expression domains during patterning of the limb. Dev Biol 157(2):410–422
Benson GV, Nguyen TH, Maas RL (1995) The expression pattern of the murine Hoxa-10 gene and the sequence recognition of its homeodomain reveal specific properties of Abdominal B-like genes. Mol Cell Biol 15(3):1591–1601
Satokata I, Benson G, Maas R (1995) Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374(6521):460–463
Rijli FM, Matyas R, Pellegrini M, Dierich A, Gruss P, Dolle P et al (1995) Cryptorchidism and homeotic transformations of spinal nerves and vertebrae in Hoxa-10 mutant mice. Proc Natl Acad Sci 92:8185–8189
Kolon TF, Wiener JS, Lewitton M, Roth DR, Edmond T. Gonzales J, Lamb DJ (1999) Analysis of homeobox gene Hoxa10 Mutations in cryptorchidism. J Urol 161:275–280
Bertini V, Bertelloni S, Valetto A, Lala R, Foresta C, Simi P (2004) Homeobox HOXA10 gene analysis in cryptorchidism. J Pediatr Endocrinol Metab 17(1):41–45
Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K et al (1995) Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 121:1373–1385
Bomgardner D, Hinton BT, Turner TT (2003) 5′ Hox Genes and Meis 1, a Hox-DNA binding cofactor, are expressed in the adult mouse epididymis. Biol Reprod 68:644–650
Lewis AG, Pecha BR, Smith EP, Gardner BJ, Hsieh-Li HM, Potter SS et al (2003) Early orchiopexy restores fertility in the Hoxa 11 gene knockout mouse. J Urol 170(1):302–305
Small KM, Potter SS (1993) Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev 7(12A):2318–2328
Moon AM, Capecchi MR (2000) Fgf8 is required for outgrowth and patterning of the limbs. Nat Genetics 26:455–459
Crossley PH, Martin GR (1995) The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121:439–451
Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M et al (2000) Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127(11):2471–2479
Shamim H, Mason I (1999) Expression of Fgf4 during early development of the chick embryo. Mech Dev 85(1–2):189–192
Miller JR (2002) The Wnts. Genome Biol 3(1):REVIEWS3001
Parr BA, Shea MJ, Vassileva G, McMahon AP (1993) Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119:247–261
Parr BA, McMahon AP (1998) Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395(6703):707–710
Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126(6):1211–1223
Miller C, Pavlova A, Sassoon DA (1998) Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 76(1–2):91–99
Author information
Authors and Affiliations
Corresponding author
Additional information
Jenny Huynh, Natalie S. Shenker and Sophie Nightingale made equal contributions to the paper.
Rights and permissions
About this article
Cite this article
Huynh, J., Shenker, N.S., Nightingale, S. et al. Signalling molecules: clues from development of the limb bud for cryptorchidism?. Pediatr Surg Int 23, 617–624 (2007). https://doi.org/10.1007/s00383-007-1907-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-007-1907-9