Abstract
In spite of the innovations in the management of newborns with congenital diaphragmatic hernia (CDH) presenting with respiratory distress at birth, mortality and ongoing morbidity still remain high. This is a retrospective analysis of newborns with CDH to determine the immediate and long-term outcomes among survivors. Medical records of newborns with CDH and respiratory distress at birth between January 1993 and March 2002 were reviewed retrospectively. There were 45 newborns, 29 males and 16 females. Eleven newborns (24%) died during the period of preoperative stabilization, 9 from pulmonary hypoplasia and 2 with complex anomalies who were not resuscitated. Surgery was performed in 34 newborns (76%). Three died postoperatively from severe pulmonary hypoplasia and pulmonary hypertension. Eleven newborns (24%) had sepsis from coagulative-negative staphylococci. Thirty-one of 43 newborns (72%) with isolated CDH were discharged home. Twenty-seven of 31 survivors (87%) had adverse long-term outcome and 2 late deaths were from pulmonary complications. Twenty-nine of 43 newborns (67%) with isolated CDH survived. The principal determinant of survival was pulmonary hypoplasia. Eighty-seven percent of survivors have associated morbidity including ongoing pulmonary, nutritional and neuro-developmental problems. Nevertheless preoperative stabilization and delayed surgery have been a satisfactory mode of management.
Similar content being viewed by others
Introduction
In spite of the recent advances in the medical and surgical management of newborns with congenital diaphragmatic hernia (CDH) presenting with respiratory distress at birth, the degree of associated pulmonary hypoplasia remains the important determinant of survival. With the management of CDH including delayed surgery, extracorporeal membrane oxygenation (ECMO), high-frequency oscillatory ventilation (HFOV), inhaled nitric oxide (NO), exogenous surfactant, partial liquid ventilation and synchronized ventilation, the survival rate ranges from 53 to 90% [1–8]. After Sakai et al. [9] reported that early surgical repair may reduce pulmonary compliance, impede mechanical ventilation and increase circulatory instability, the treatment of CDH is no longer considered a surgical emergency. Since the early 1990s delayed surgery has been the accepted management of newborns with CDH at King Fahad National Guard Hospital, Riyadh, Saudi Arabia [1].
The medical literature is replete with information on immediate outcome of CDH survivors, but is sparse on the long-term sequelae. However, there are reports that 12–61% of long-term survivors have continuing problems including pulmonary function, nutrition and growth, neuro-developmental and musculoskeletal systems [10–14].
The aim of this study is to evaluate the immediate and long-term outcomes of all newborns with CDH and respiratory distress at birth, who were managed at this hospital.
Materials and methods
The medical charts of newborns with CDH and respiratory distress at birth who were treated at King Fahad National Guard Hospital, Riyadh, between January 1993 and March 2002 were retrospectively analyzed. The data retrieved included age, gender, birth weight and gestational age, place of birth, prenatal diagnosis, site of defect, Apgar scores, timing of surgery, management and outcome.
Results
There were 45 CDH newborns, 29 males and 16 females. Thirty-six newborns (80%) were delivered in this hospital and 20/36 (56%) were diagnosed in utero. One of the 19 newborns who were transferred from other institutions was diagnosed by prenatal ultrasound. The birth weight of newborns with CDH ranged from 1.10 to 3.80 kg (mean 2.98 kg) and the mean gestational age was 37 weeks (range 30–41 week). The mean Apgar scores at 1 min and 5 min were 4 (range 0–9) and 7 (range 1–9), respectively. Thirty-seven of the 45 newborns (82%) had left-sided CDH. All newborns were either intubated in the delivery room or in neonatal intensive care unit (NICU) and were sedated, paralyzed and ventilated. The aim of ventilatory support was to maintain the blood pH between 7.45 and 7.55, the PaO2 above 100 mmHg, the SaO2 above 96% and PaCO2 between 25 and 35 mmHg. The preoperative management has already been reported [1]. Since 2003, permissive hypercapnia has been used instead of hyperventilation; however, none of the patients were included in this review. Antibiotics were administered preoperatively and continued postoperatively for 2–7 days.
Immediate outcome
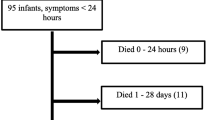
During the period of preoperative stabilization, 11 newborns (24%) died, 6 within 24 h. Nine died from pulmonary hypoplasia and persistent pulmonary hypertension and two premature infants, 30 and 34 weeks’ gestation, were not resuscitated because of severe associated congenital anomalies: one with chromosomal abnormality, agenesis of corpus callosum and severe hypoplastic lungs; and the other with cleft lip and palate, atrophic right thumb, holoprosencephaly and dilated left ventricle. Five of 11 CDH newborns (45%) that died preoperatively were diagnosed by prenatal ultrasound. Because of persistent pulmonary hypertension, 13 newborns were shifted to HFOV, 2 required inhaled NO and 7 were given exogenous surfactant. Newborns who were hemodynamically stable, had the acidosis corrected and their oxygenation was satisfactory while on conventional ventilator for at least 24 h were considered ready for surgical repair.
Thirty-four newborns (76%) had surgical repair between 1 and 26 days (mean 4 days). Repair was via laparotomy in 33 (97%) and through thoracotomy in 1 right-sided CDH. Primary repair was possible in 29 (75%) newborns, but Gore-Tex patches were required in 5 in whom it was not possible. A hernia sac was present in 9 out of 34 newborns (26%). Thirteen newborns had prophylactic ipsilateral postoperative chest tube inserted to free drainage but the practice was discontinued in 1999.
Three newborns that required HFOV preoperatively died 5–23 days (mean 12.6 days) after surgery from pulmonary hypoplasia and associated pulmonary hypertension. Seven newborns developed gastroesophageal reflux (GER) and were managed by anti-reflux measures including anti-reflux medications. One required Nissen fundoplication with feeding gastrostomy because of persistent vomiting and poor weight gain. Eleven of 45 newborns (24%) had coagulase-negative Staphylococcus sepsis proven by blood culture. The mean period of ventilation was 16 days (range 3–53 days) and the length of hospital stay ranged from 9 to 122 days (mean 36.5 days). Thirty-one of 43 newborns (72%) with isolated CDH survived to be discharged home.
Long-term outcome
The age at review of long-term outcome of 31 CDH survivors ranged from 6 months to 9 years (Table 1). Four survivors were free of morbidity. Twenty-seven of 31 survivors (87%), however, had ongoing adverse outcome including pulmonary, nutritional and growth, neuro-developmental, musculoskeletal problems and further medical and surgical management. Because of the lack of coordinated follow up, clinical information was incomplete in six children.
Fourteen children (45%) had recurrent wheezing attacks and required inhaled bronchodilators and/or steroids. Three children were admitted frequently to hospital for severe wheezing attacks and/or pneumonia. A child with severe right pulmonary hypoplasia and postpneumonectomy-like syndrome underwent thoracotomy with insertion of right hemithorax prosthesis and tracheostomy. Despite these procedures, his respiratory symptoms did not improve requiring supplemental oxygen at home. He died, when 2 years old, from respiratory failure. Another child, 9 months old, died from respiratory complications following repair of recurrent diaphragmatic hernia.
Eight children (18%) had GER and were treated with anti-reflux measures including medications, but two required Nissen fundoplication for failure of medical treatment, persistent vomiting and respiratory symptoms. Failure to thrive (FTT), a weight below the 5th percentile, was present in seven children (23%). They included a child with GER who gained weight after fundoplication and two children who were having chronic respiratory problems.
Significant neuro-developmental problems affected seven survivors. Four had global developmental delay either alone or associated with speech and hearing impairment, but no cerebral palsy. A child with a family history of speech impairment had the same defect. Two children having hearing impairment received hearing aids at the ages of 2 and 5 years, and a child with myopia has had eyeglasses for correction.
Musculoskeletal problems of the chest wall included pectus excavatum in three children who are being followed up in the clinic and a child with scoliosis who wears a thoracic brace. Three children developed first-time recurrences of diaphragmatic hernia; two right-sided and one left. Both right recurrences were repaired via thoracotomy using prosthetic patches, but one recurred again and was repaired through an abdominal approach. Through laparotomy the left recurrence was repaired without using a prosthetic patch. The recurrences occurred from 3 to 15 months with a mean of 5.8 months. The clinical presentation included cough, vomiting and signs of intestinal obstruction.
Seven children were admitted with adhesive small bowel obstruction between the ages of 2 months and 7 years. Three required laparotomy and release of adhesions after failure of non-operative management. The other types of surgery performed on CDH children included Spigelian hernia repair (1), orchiopexy (2) and myringotomy (1).
Discussion
Since the 1980s delayed surgery has become an accepted method for the treatment of CDH after Sakai et al. [9] reported that early surgical repair worsened the cardiopulmonary function. Although improved survival of up to 90% has been reported in some centers using delayed surgery [1–3], this has not been the experience of other groups [5, 15]. Delayed surgery allows for stabilization of CDH newborns with pulmonary hypoplasia and associated pulmonary hypertension and also avoids surgery in newborns that are most likely to die. Reickert et al. [2] and Boloker et al. [8] reported that with the mean age at repair of 8.9 and 4.5 days, respectively, survival improved without increasing morbidity with less demand for ECMO. The optimal timing of surgery is still not universally accepted. Recently, Rozmiarek et al. [16] reported that cardiac defects, renal failure and the initial blood gases are the significant factors that influence survival and not the timing of surgery. Therefore to improve the outcome of CDH newborns, timing of surgery should be based on optimizing these clinical parameters as opposed to a specific time period. This conclusion supports our policy to delay surgery until the newborn is hemodynamically stable, the acidosis corrected and the oxygenation satisfactory and, furthermore, explains why 11/45 (24%) newborns were never taken to surgery. In this series of delayed surgery, the survival rate of 67% in newborns with isolated CDH compares favorably with the results from other institutions [2, 8, 10, 16–18].
Twelve (86%) early deaths (before discharge) were from pulmonary hypoplasia and associated pulmonary hypertension and two from lethal complex anomalies. Thus, pulmonary hypoplasia is the major determinant of survival and also explains why, despite all the new therapies available to treat pulmonary hypotension, the mortality rate with CDH has not changed [19]. Seven of 13 newborns (54%) died despite the use of HFOV. These were very sick patients with overwhelming pulmonary hypoplasia and associated hypertension. The role of HFOV in CDH remains uncertain because of conflicting results [4, 20, 21]. Inhaled NO was given to only two patients and the benefit in the management of CDH in this series is uncertain. Inhaled NO for CDH newborns has been associated with good outcome in some centers, but other groups have had disappointing results [5, 19, 20]. Exogenous surfactant use has had satisfactory reports from various institutions but is not accepted worldwide [6, 8, 22]. The role of these therapeutic agents is still uncertain and prospective controlled trials are required for clarification. Reports of combination of different modalities of treatment have shown a good survival rate from 76 to 96% [8, 23, 24].
Sepsis in newborns with CDH is rarely reported. Staphylococci are a common cause of hospital-acquired infections in many NICUs [25]. Al-Hathal et al. [1] reported coagulase-negative staphylococci as the causative organism in approximately 82% of CDH patients with proven sepsis. In this series, the same organism was the cause of sepsis in 24% of newborns with CDH. The risk factors for staphylococcal infection in newborns in NICU include: birth weight; illness; presence of indwelling catheters such as central venous catheter (CVC), chest tubes and endotracheal intubation; and the length of hospital stay. The risk has been shown to increase if the CVC is used to administer parenteral nutrition, especially lipids [26]. Although perioperative course of antibiotics is given to CDH patients, careful handling of patients is necessary to minimize the risk of sepsis. Strict use of sterile techniques with procedures such as insertion of CVCs, insertion of chest tubes, endotracheal intubations and suctioning and hand washing may reduce the incidence of sepsis in CDH patients in the NICU.
This study reveals ongoing morbidity among 27/31 survivors (87%) including adverse pulmonary, nutritional and growth, neuro-developmental and musculoskeletal outcome and/or required further surgical management. Ongoing problems in CDH survivors have been reported as 12%–61% and depend on the duration of follow-up [10–14, 27].
Pulmonary problems are a major source of morbidity in CDH survivors. The problems may be due to a combination of factors: the degree of pulmonary hypoplasia; iatrogenic barotrauma; oxygen toxicity; recurrent pneumonias; chronic lung disease; hyperactive airway disease; scoliosis; pectus excavatum; and chronic aspiration from GER. Approximately 45% of our long-term survivors had chronic respiratory symptoms such as hyperactive airway disease and required inhaled bronchodilators and/or steroids. Due to ineffective breathing, CDH children are admitted frequently to hospital with recurrent pneumonias and/or hyperactive airway disease [20, 28], and this occurred in three children in this series. Stefanutti et al. [28] have reported that the presence of residual lung hypoplasia in several long-term CDH survivors, and the initial ventilatory management may contribute to pulmonary morbidity. Pulmonary morbidity may continue in some children for many years after repair and in this series resulted in two late deaths. Muratore et al. [14] reported that 16% of their survivors required supplemental oxygen at discharge, but this was not our experience.
Nutritional and growth problems are particularly common in the first year of life of CDH infants [11, 27]. Fourteen of 31 survivors (45%) had nutritional and growth problems. Seven children (23%) had a weight below the 5th percentile, which is within the range of 20–40% reported by Jaillard et al. [13]. Persistent gastrointestinal, respiratory symptoms or food aversion may contribute to growth failure in CDH survivors [12, 13, 20, 29]. Two of seven children (29%) with FTT were having chronic respiratory problems but there was no obvious cause in four children. The last child with GER gained weight after fundoplication. The incidence of GER in CDH survivors ranges from 12 to 69% [29, 30]. Seven newborns developed GER soon after the repair of CDH but only one required surgery. Eight of 31 survivors (26%) had GER with 2/8 (25%) needing fundoplication. Therefore most CDH survivors with GER can be managed by anti-reflux measures and medication. Factors that contribute to GER include weak diaphragmatic crura, hiatus hernia, increased intra-abdominal pressure, ectasia of the esophagus shortening of intra-abdominal esophagus, and disruption of the angle of His [1]. Jaillard et al. [13] and Muratore et al. [27] reported food aversion in 7 and 33% of their patients, respectively, with gastrostomy being required for feeding. In this series, one newborn required a feeding gastrostomy to increase calorie intake whilst undergoing fundoplication for GER but not for food aversion. Food aversion is not a problem in this series.
Neuro-developmental outcome is a concern in all CDH children, especially after the use of ECMO [20]. Nearly a quarter of the infants in this series had neuro-developmental problems of speech delay, developmental delay, and hearing loss. Jaillard et al. [13] found neurological examination to be normal at 2 years of age in 45/51 CDH children (88%), however 6 had cerebral palsy or developmental delay irrespective of the use of ECMO or not. McGahren et al. [31], however, reported significant evidence of neurological delay in 67% of CDH patients who required ECMO compared with 24% in a non-ECMO group. Contributory factors to developmental delay include: a primary neurological disorder; the result of the severity of CHD; the degree and duration of acidosis and hypoxia; and the type of treatment particularly ECMO. In this series four children had global developmental delay in addition to speech and or hearing loss. Follow-up studies have revealed hearing loss in 20–40% of CDH survivors [32, 33]. In this series 2/31 children (6%) have hearing loss and wear hearing aids. Because of the lack of coordinated follow-up, it is possible that not all patients with hearing loss have been diagnosed. Rasheed et al. [33] found hearing loss in six of six survivors (100%) stabilized with ECMO compared to two of nine (22%) of those needing ECMO postoperatively [33]. The risk factors for hearing loss in CDH children include the use of ototoxic medications such as aminoglycosides and diuretics, HFOV, ECMO, hyperventilation and pancuronium, hypoxia, prolonged mechanical ventilation, family history and other factors [10, 11,32]. Regular hearing screening of CDH survivors is necessary to pick up patients with sensorineural hearing loss.
Pectus and scoliosis are chest wall deformities that may occur in CDH children and persist into adulthood [11, 12]. Falconer et al. [34] reported an incidence of 21 and 11% of pectus excavatum and scoliosis, respectively, in survivors of CDH. In this series, three children with pectus excavatum have been followed up in the clinic with none requiring surgical treatment. A child with scoliosis wears a thoracic brace. These chest wall deformities may be related to diaphragmatic tension at closure of the defect or to the smaller thoracic cavity and smaller lung in the affected side [11, 12].
In this series diaphragmatic hernia recurrence rate is 12%, which is within the reported range of 5–50% [11, 35]. Although prosthetic patches were used for primary closure in 5/34 newborns (15%), none recurred. However, repair of a recurrence using prosthetic patch failed. In centers where prosthetic patches and ECMO are used routinely, recurrence rate is on the increase [35]. There are concerns about patch durability over the long term, and we believe that primary repair or the use of muscle flaps such as latissimus dorsi, split abdominal wall muscle flaps or pedicle flap of abdominal muscle may be undertaken whenever possible [36]. Patch repair should be reserved for the severe CDH defect and agenesis. Although our patients with recurrent CDH were symptomatic, some recurrences may be asymptomatic and may be found on routine chest X-rays. Therefore serial X-ray examination of the chest, especially those with patch repair, should be recommended [11, 36].
Survivors of CDH may require surgery for small bowel obstruction [11]. Seven children (23%) presented with adhesive small bowel obstruction, three of the seven (43%) requiring laparotomy. Other surgical procedures included orchiopexy, inguinal herniotomy and Spigelian hernia repair.
Since this study is a retrospective review of medical records, it has its flaws. These include lack of coordinated follow-up and inadequate charting details. Furthermore, our patients may have been treated at other hospitals. Autopsies were not performed and so the cause of mortality was based on the clinical findings.
In conclusion, delayed surgery is a satisfactory method of management for isolated CDH newborns with an overall survival rate of 67%. The most common cause of mortality is severe pulmonary hypoplasia. Long-term survivors have significant continuing medical and surgical problems and late deaths can occur from pulmonary complications. Coordinated multidisciplinary follow-up of CDH survivors in our hospital is essential to detect morbidity early and to improve the long-term outcome.
References
Al-Hathal M, Crankson SJ, Al-Harbi F, Ahmed G, Tawil K (1998) Congenital diaphragmatic hernia: experience with preoperative stabilization and delayed surgery without ECMO and initialed nitric oxide. Am J Perinat 15:487–490
Reickert CA, Hirschl RB, Schumacher R, et al (1996) Effect of very delayed repair of congenital diaphragmatic hernia on survival and extracorporeal life support use. Surgery 120:766–773
Weber TR, Kountzman B, Dillon PA, Silen ML (1998) Improved survival in congenital diaphragmatic hernia with evolving therapeutic strategies. Arch Surg 133:498–502
Desfrere L, Jarreau PH, Dommergues M, et al (2000) Impact of delayed repair and elective high-frequency oscillatory ventilation on survival of antenatally diagnosed congenital diaphragmatic hernia: first application of these strategies in the more “severe” subgroup of antenatally diagnosed newborns. Intensive Care Med 26:934–941
Okuyama H, Kubota A, Oue T, et al (2002) Inhaled nitric oxide with early surgery improves the outcome of antenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg 37:1188–1190
Glick PL, Leach CL, Besner GE, et al (1992) Pathophysiology of congenital diaphragmatic hernia 111:exogenous surfactant therapy for the high-risk neonate with CDH. J Pediatr Surg 27:866–869
Fauza DO, Hirschl RB, Wilson JM (2001) Continuous intrapulmonary distension with perfluorocarbon accelerates lung growth in infants with congenital diaphragmatic hernia: initial experience. J Pediatr Surg 36:1237–1240
Boloker J, Bateman DA, Wung JT, Stolar CJH (2002) Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg 37:357–366
Sakai H, Tamura H, Hosokawa Y, Bryan AC, Barker GA, Bohn DJ (1987) Effect of surgical repair on respiratory mechanics in congenital diaphragmatic hernia. J Pediatr 111:432–438
Bagolan P, Casaccia G, Crescenzi F, Nahom A, Trucchi A, Giorlandino C (2004) Impact of a current treatment protocol on outcome of high-risk congenital diaphragmatic hernia. J Pediatr Surg 39:313–318
Lund DP, Mitchell J, Kharasch V, Quigley S, Kuehn M, Wilson JM (1994) Congenital diaphragmatic hernia: the hidden morbidity. J Pediatr Surg 29:258–264
Huddy CLJ, Boyd PA, Wilkinson AR, Chamberlain P (1999) Congenital diaphragmatic hernia: prenatal diagnosis, outcome and continuing morbidity in survivors. Br J Obstet Gynaecol 106:1192–1196
Jaillard SM, Pierrat V, Dubois A, Truffert P, et al (2003) Outcome at 2 years of infants with congenital diaphragmatic hernia: a population-based study. Ann Thoracic Surg 75:250–256
Muratore CS, Kharasch V, Lund DP, et al (2001) Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J Pediatr Surg 36:133–140
Moyer V, Moya F, Tibboel R, Losty P, Nagaya M, Lally KP (2002) Late versus early surgical correction for congenital diaphragmatic hernia in newborn infants. Cochrane Database Syst Rev 3:CD 001695
Rozmiarek AJ, Qureshi FG, Cassidy L, Ford HR, Hackam DJ (2004) Factors influencing survival in newborns with congenital diaphragmatic hernia: the relative role of timing of surgery. J Pediatr Surg 39:821–824
Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ (1995) Congenital diaphragmatic hernia: survival treated with delayed surgery, spontaneous respiration and no chest tube. J Pediatr Surg 30:406–409
Langham MJ Jr, Kays DW, Beierle EA (2003) Twenty years of progress in congenital diaphragmatic hernia at the University of Florida. Am Surg 69:45–52
Stege G, Fenton A, Jaffray B (2003) Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 112:532–535
Davis PJ, Firmin RK, Manktelow B, et al (2004) Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr 144:309–315
Azarow K, Messineo A, Pearl R, Filler R, Barker G, Bohn D (1997) Congenital diaphragmatic hernia—a tale of two cities: the Toronto experience. J Pediatr Surg 32:395–400
Van Meurs K; Congenital Diaphragmatic Hernia Study Group (2004) Is surfactant therapy beneficial in the treatment of the term newborn infants with congenital diaphragmatic hernia? J Pediatr 145:312–316
Somaschini M, Locatelli G, Salvoni L, Bellan C, Colombo A (1999) Impact of new treatments for respiratory failure on outcome of infants with congenital diaphragmatic hernia. Eur J Pediatr 158:780–784
Osiovich HC (2004) Improving survival of neonates with isolated congenital diaphragmatic hernia. Indian Pediatr 41:1138–1142
St Geme JW, Harris MC (1991) Coagulase-negative staphylococcal infection in the neonate. Clin Perinatol 18:281–302
Freeman J, Goldmann DA, Smith NE, Sidebottom DG, Epstein MF, Platt R (1990) Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N Engl J Med 323:301–308
Muratore CS, Utter S, Jaksic T, Lund DP, Wilson JM (2001). Nutritional morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg 36:1171–1176
Stefanutti G, Filippone M, Tommasoni N, et al (2004). Cardiopulmonary anatomy and function in long-term survivors of mild to moderate congenital diaphragmatic hernia. J Pediatr Surg 39:526–531
Nagaya M, Akatsuka H, Kato J (1994) Gastro esophageal reflux occurring after repair of congenital diaphragmatic hernia. J Pediatr Surg 29:1447–1451
Stolar CJH, Levy DP, Dillon PW, Reyes C, Belamarich P, Berdon WE (1990) Anatomic and functional abnormalities of the esophagus in infants surviving congenital diaphragmatic hernia. Am J Surg 159:204–207
McGahren ED, Mallik K, Rodgers BM (1997) Neurological outcome is diminished in survivors of congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation. J Pediatr Surg 32:1216–1220
Kuga T, Taniguchi S, Inoue T, Zempo N, Esato K (2000) Hearing loss in infants with congenital diaphragmatic hernia treated without extracorporeal membrane oxygenation: report of two cases. J Pediatr Surg 35:621–623
Rasheed A, Tindall S, Cueny DL, Klein MD, Delaney-Black V (2001) Neuro-developmental outcome after congenital diaphragmatic hernia: extracorporeal membrane oxygenation before and after surgery. J Pediatr Surg 36:539–544
Falconer AR, Brown RA, Helms P, Gordon I, Baron JA (1990) Pulmonary sequelae in survivors of congenital diaphragmatic hernia. Thorax 45:126–129
Sydorak RM, Hoffman W, Lee H, et al (2003) Reversed latissimus dorsi muscle flap for repair of recurrent congenital diaphragmatic hernia. J Pediatr Surg 38:296–300
Moss RL, Chen MC, Harrison MR (2001) Prosthetic patch durability in congenital diaphragmatic hernia: a long-term follow-up study. J Pediatr Surg 36:152–154
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crankson, S.J., Al Jadaan, S.A., Namshan, M.A. et al. The immediate and long-term outcomes of newborns with congenital diaphragmatic hernia. Ped Surgery Int 22, 335–340 (2006). https://doi.org/10.1007/s00383-006-1643-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-006-1643-6