Abstract
Purpose
Nonsyndromic craniosynostosis (NSC) is associated with neurocognitive deficits, and intervention at infancy is standard of care to limit the negative effects of NSC on brain development. In this study, diffusion tensor imaging (DTI) was implemented to investigate white matter microstructure in infants with NSC undergoing cranial vault remodeling, and a comparison was made with white matter development in neurotypical controls.
Methods
Infants presenting with NSC (n = 12) underwent DTI scans before and after cranial vault remodeling. Neurotypical infants (n = 5), age matched to NSC patients at preoperative scans, were compared to preoperative DTI scans. Pre- and postoperative NSC scans were compared in aggregate, and the sagittal synostosis (n = 8) patients were evaluated separately. Finally, neurotypical infants from the University of North Carolina/University of New Mexico Baby Connectome Project (BCP), who underwent DTI scans at timepoints matching the NSC pre- and postoperative DTI scans, were analyzed (n = 9). Trends over the same time period were compared between NSC and BCP scans.
Results
No significant differences were found between preoperative NSC scans and controls. White matter development was more limited in NSC patients than in BCP patients, with microstructural parameters of the corpus body and genu and inferior and superior longitudinal fasciculi consistently lagging behind developmental changes observed in healthy patients.
Conclusion
Infant white matter development appears more limited in NSC patients undergoing cranial vault remodeling relative to that in neurotypical controls. Further investigation is needed to explore these differences and the specific effects of early surgical intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonsyndromic craniosynostosis (NSC) has an incidence of 0.4 to 1 in 1000 live births [1] and is defined at our institution as any single-suture craniosynostosis in the absence of additional anomalies of the face, trunk, or limbs. NSC is associated with a higher prevalence of neurocognitive impairments relative to the general population, hypothesized to result from abnormal brain activity related to the premature fusion of the cranial sutures [2].
Children with NSC have higher rates of cognitive, language, and motor disabilities across all suture subtypes [3, 4]. Additionally, deficiencies in visual spatial programming organization, fixation of visual attention, writing competence, inhibitory control, and divided attention have been identified [5, 6]. While the precise nature of the relationship between NSC and these neurocognitive deficits is unclear, in metopic synostosis specifically, a more severe phenotype has been correlated with worse neurocognitive outcomes, suggesting that this relationship may exist on a continuum [7].
The effects of surgical correction of NSC reported in the literature have been variable. Surgically corrected single-suture craniosynostosis patients have demonstrated distinct neuroanatomical differences compared to controls, including differences in the volume of the ventricles, corpus callosum, and cerebellar vermis [8]. However, fMRI studies have identified normalization of aberrant connectivity in the auditory network, V2 and V3 visual networks, default mode network, and left frontoparietal network after surgical intervention in sagittal NSC [9]. Additionally, surgical correction of sagittal NSC has been associated with normalized brain growth and morphology [10]. Studies have demonstrated improved neurocognitive outcomes with earlier surgical intervention and potential improvements of developmental delay in late-presenting infants with NSC [11, 12].
Given the limited existing data, further investigation is needed to better characterize the effects of surgical correction of NSC on brain development. Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) modality that measures the directional diffusion of water molecules to visualize white matter connections between different brain regions [13]. DTI parameters change in a well-characterized way in early development and reflect microstructural white matter changes such as degree of myelination and axonal diameter, and abnormalities in these parameters have been linked to conditions including cerebral visual impairment and states of emotional dysregulation [14,15,16]. In this study, we use DTI to assess white matter development in children with NSC undergoing cranial vault remodeling compared to healthy controls.
Methods
Patient population and group comparisons
This study was performed in accordance with the Yale Institutional Review Board (HIC # 2000030969). Patients with NSC recruited between 2017 and 2023 underwent MRI analysis without anesthesia before surgical intervention and 8–12 weeks postoperatively. All patients underwent open cranial vault remodeling, which consists of complete removal of the skull for reshaping. Each MRI session included enough time for the infants to be put back to sleep if awoken and to complete the MRI scan sequence lasting 30–45 min. In the postoperative period, parents could elect to pursue a helmet, but this was done only at parental request and no earlier than 4 weeks postoperatively. All infants who completed the DTI scans were included in our analysis. Infants without NSC were recruited as part of a previous study at our institution and underwent MRI analysis between 2014 and 2017; these controls were only scanned at one timepoint, and scans were age matched to preoperative NSC scans. Because these controls only provided a comparison to preoperative scans, data from neurotypical patients enrolled in the University of North Carolina/University of New Mexico Baby Connectome Project (BCP) were analyzed as further control data. BCP scans corresponding to the average pre- and postoperative ages of NSC patient scans were utilized as a comparison to typical brain development, and all BCP patients analyzed were scanned at both corresponding timepoints.
Four groups were analyzed: the NSC cohort, the sagittal suture subset of the NSC cohort, healthy controls matched to preoperative age, and the BCP cohort. The sagittal suture subset of the NSC cohort included patients with sagittal synostosis or combined sagittal and metopic synostosis. For patients in the full NSC cohort and the sagittal suture subset, preoperative and postoperative DTI scans were compared. The full NSC cohort and sagittal suture subset patients were compared directly to control patient scans age matched to preoperative NSC scans. Finally, developmental changes between pre- and postoperative scans of both NSC patient cohorts were compared to those of the BCP cohort to analyze neuroanatomical changes over the same time period.
Imaging parameters
All MRI scans were obtained using a single 3-T Siemens Tim Trio MR system with a 32-coil polarized head coil. The DTI protocol consists of a localizer scan, an MP RAGE anatomical scan, and one to three repetitions of the diffusion-weighted sequence. DTI data were obtained using two different sequences: sequence1 included 30 diffusion gradient directions and five b = 0 images with TE = 86, TR = 6400, 96 × 96 acquisition matrix, bandwidth 2367, flip angle = 90, and FOV = 240 × 240 mm, with 55 slices, 2.5 mm thick; in sequence2, two transverse DTI datasets with opposite polarity of the PE direction (i.e., PA and AP) were acquired, each consisting of two b = 0 images, 99 diffusion gradient directions with TE = 89.2, TR = 3230, 140 × 140 acquisition matrix, bandwidth 1700, flip angle 78, and FOV = 210 × 210 mm, with 92 slices, 1.5 mm thick, skip 0 mm. Six subjects completed both pre- and postoperative scans with sequence1, and six subjects completed both pre- and postoperative scans with sequence2. We also analyzed nine BCP subjects with 4–6-month and 7–9-month DTI scans. The BCP sequence was as follows: two transverse DTI datasets with opposite polarity of the PE direction (i.e., PA and AP) were acquired, each consisting of 78 diffusion gradient directions and two b = 0 images with TE = 88.6, TR = 2640, 140 × 140 acquisition matrix, flip angle = 78, and FOV = 210 × 210 mm, with 92 slices, 1.5 mm thick.
DTI analysis
The FMRIB Software Library (FSL) was used to pre-process DTI datasets. Binary masks were made using the BET tool. To analyze sequence2 and the BCP sequence, TOPUP was used to correct for susceptibility artifacts where volumes acquired with opposing phase-encoding directions were combined and corrected for artifacts of motion and eddy currents using EDDY. For sequence1, only EDDY was used for motion correction. For both sequences, the corrected diffusion-weighted dataset, binary mask, b-values, and b-vectors were then used in dtifit to output the four scalar images of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). Broadly, FA measures the overall directionality of molecular diffusion, MD measures the overall molecular diffusion rate, AD measures the molecular diffusion rate along the main diffusion axis, and RD measures the molecular diffusion rate transverse to the main axis [17]. Physiological associations of these scalars are described in the discussion below. Each subject’s FA map was non-linearly registered to the EBDS Infant DTI Fiber Atlas FA map using Bioimage Suite, and then, all subject scalar maps were resliced to the EBDS infant template brain space to run paired t-tests between pre- and postoperative scans in AFNI. To correct for multiple comparisons, we used FWE correction determined by Monte Carlo simulation using the AFNI 3dClustSim program. Two different significance thresholds were applied for different DTI measures, and those thresholds are specified within each figure legend.
Results
Patient characteristics
Twelve NSC patients underwent MRI for DTI measurements before and after surgical intervention. The distribution of suture subtypes was 42% sagittal (n = 5), 25% sagittal/metopic (n = 3), 17% metopic (n = 2), and 17% unicoronal (n = 2). The mean patient age at surgery was 5.4 ± 1.1 months, and all patients underwent cranial vault remodeling. Pre- and postoperative scans were taken at 4.6 ± 1.5 months (0.8 ± 0.6 months prior to surgery) and 8.1 ± 1.6 months (2.6 ± 0.9 months following surgery), respectively. In patients with sagittal or sagittal/metopic synostosis (n = 8), the average age at pre- and postoperative measurements was 4.5 ± 1.8 months and 8.2 ± 1.9 months, respectively. Five control infants (mean age = 5.0 ± 1.4 months) without craniosynostosis underwent DTI measurements.
Data from nine patients with no neurological or craniofacial abnormalities, enrolled in the University of North Carolina/University of New Mexico BCP, were analyzed. Two chronological DTI scans (mean age = 5.1 ± 0.8 months and 8.1 ± 0.8 months) were used for each patient.
Preoperative NSC scans vs. age-matched controls
No significant differences were found between the sagittal suture subset (n = 8) or the full NSC cohort preoperatively (n = 12) and age-matched controls (n = 5) on any of the measures studied (FA, MD, AD, and RD) at a significance level of P < 0.001.
Preoperative vs. postoperative NSC scans and 5-month vs. 8-month baby connectome scans
FA
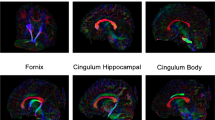
FA changed extensively between 5- and 8-month BCP scans; specific tracts included the corpus callosum, forceps major and minor, superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), and corona radiata (P < 0.0001, Fig. 1a, Table 1, Online Resource 1). FA increased in all significant tracts at the 8-month scan relative to the 5-month scan. The sagittal suture subset saw more limited changes in FA (P < 0.001, Fig. 1b, Table 1, Online Resource 2) relative to the BCP patients. Whereas the corpus body and genu, SLF, ILF, and brainstem had no changes in FA before and after surgery in sagittal NSC patients, these regions increased in FA in BCP patients over the equivalent time span. In the full NSC cohort, FA did not change in the corpus body, ILF, or brainstem (P < 0.001, Fig. 1c, Table 1, Online Resource 3). The full NSC cohort did, however, show an increase in FA in the temporal radiations, which was not observed in the BCP cohort.
Coloration shows white matter tracts with significant FA changes across different timepoints for specific patient groups: a changes between 5 and 8 months in the BCP cohort (n = 9, P < 0.0001); b changes between pre- and postoperative scans in the sagittal suture subset of the NSC cohort (n = 8, P < 0.001); c changes between pre- and postoperative scans in the full NSC cohort (n = 12, P < 0.001)
MD
BCP scans showed widespread decreases in MD over time. Specific tracts with changes included the corpus callosum, forceps major and minor, SLF, ILF, inferior fronto-occipital fasciculus (IFOF), internal capsule, and corona radiata (P < 0.0001, Fig. 2a, Table 2, Online Resource 1). Changes in MD were more limited in the sagittal suture subset compared to those in the BCP cohort (P < 0.001, Fig. 2b, Table 2, Online Resource 2). Pre- and postoperative comparison showed no change in the corpus body or genu, SLF, IFOF, or internal capsule, all of which showed a decrease in MD in BCP patient scans over the equivalent time span. MD also decreased in the cerebellum and temporal cingulum in the sagittal cohort but not the BCP cohort. In the full NSC cohort, MD did not change in the corpus body or genu; it did change in these locations in the BCP cohort (P < 0.0001, Fig. 2c, Table 2, Online Resource 3). Additionally, MD decreased in the cerebellum, temporal radiations, and inferior frontal gyrus radiations in NSC but not BCP infants.
Coloration shows white matter tracts with significant MD changes across different timepoints for specific patient groups: a changes between 5 and 8 months in the BCP cohort (n = 9, P < 0.0001); b changes between pre- and postoperative scans in the sagittal suture subset of NSC cohort (n = 8, P < 0.001); c changes between pre- and postoperative scans in the full NSC cohort (n = 12, P < 0.0001)
AD and RD
BCP scans showed fairly limited changes in AD between 5 and 8 months (P < 0.0001, Fig. 3a, Table 3, Online Resource 1). AD decreased in the corpus splenium, SLF, ILF, posterior IFOF (PIFOF), and corona radiata. Both the sagittal suture subset and complete NSC cohort showed more extensive changes, with postoperative scans showing AD decreases in the forceps minor and major and internal capsule, none of which were seen in BCP patients (P < 0.001, Fig. 3b, c, Table 3, Online Resources 2 and 3). In the complete NSC cohort, AD also decreased in the cerebellum and occipital pole, but not in the BCP cohort. Finally, in the sagittal but not the BCP cohort, AD decreased in the cerebellar peduncle, temporal projections, occipital projections, and stria terminalis. RD showed more extensive decreases than AD in 8-month BCP scans, including decreases in all regions of the corpus callosum, forceps minor and major, SLF, ILF, PIFOF, corona radiata, internal capsule, and cerebellum (P < 0.0001, Fig. 4a, Table 4, Online Resource 1). Sagittal cohort and complete NSC cohort scans showed similar changes in RD postoperatively compared to the BCP cohort (P < 0.001, Fig. 4b, and P < 0.0001, Fig. 4c, Table 4, Online Resources 2 and 3). Notably, RD did not decrease postoperatively in the corpus genu or body for either group. Additionally, RD in the ILF did not change in the complete cohort. Finally, in the complete NSC cohort but not the BCP cohort, AD decreased in the brainstem, motor projections, occipital projections, and frontal projections.
Coloration shows white matter tracts with significant AD changes across different timepoints for specific patient groups: a changes between 5 and 8 months in the BCP cohort (n = 9, P < 0.0001); b changes between pre- and postoperative scans in the sagittal suture subset of the NSC cohort (n = 8, P < 0.001); c changes between pre- and postoperative scans in the full NSC cohort (n = 12, P < 0.001)
Coloration shows white matter tracts with significant RD changes across different timepoints for specific patient groups: a changes between 5 and 8 months in the BCP cohort (n = 9, P < 0.0001); b changes between pre- and postoperative scans in the sagittal suture subset of the NSC cohort (n = 8, P < 0.001); c changes between pre- and postoperative scans in the full NSC cohort (n = 12, P < 0.0001)
Discussion
This study characterized changes in white matter tract microstructure in infants with NSC who underwent cranial vault remodeling and compared white matter development to neurotypical infants. Neither sagittal suture subset nor full NSC cohort preoperative scans showed significant differences in white matter parameters compared to neurotypical, age-matched controls. Comparison of pre- and postoperative scans showed more limited white matter development in the complete NSC cohort compared to changes observed over the same age span in BCP scans. Sagittal subset patients showed the greatest reduction in white matter development relative to the BCP cohort, with major commissural and association tracts not showing expected microstructural changes over time.
The normal development of white matter through infancy and early childhood has been well characterized [18,19,20]. In general, FA and MD change extensively in major white matter tracts during the first 2 years of life and reach near-adult values between 24 and 48 months of age. While FA values for these tracts are observed to increase, MD values decrease, likely reflecting increasing axonal diameter, increasing myelination of white matter, tighter packing of nerve fibers, and loss of water from the brain [21]. AD and RD, other measures of diffusivity, also typically decrease over this early time [22]. Analysis of patients in the BCP cohort revealed trends largely consistent with expectations for the development of major white matter tracts in neurotypical infants from 5 to 8 months of age.
There is a dearth of literature describing the effects of NSC on early white matter development. A recent study comparing preoperative metopic NSC patients with controls found no significant difference in white matter microstructure in the frontal lobe [23]. However, patients with syndromic craniosynostosis, which is generally more severe than NSC, have been shown to have microstructural white matter disturbances despite prior surgical correction of skull deformity [24, 25].
The current study found no significant differences in white matter microstructure between scans of preoperative NSC patients and age-matched controls in both the sagittal-only subset and full NSC cohort. While this finding suggests that the presence of NSC may not cause major disturbances in the white matter microstructure prior to surgical correction, the analysis comparing preoperative scans (complete cohort: n = 12; sagittal subset: n = 8) to age-matched controls (n = 5) likely had less power to detect differences than the within-group comparisons, which had greater sample sizes and utilized paired statistical testing.
Comparison of white matter development in pre- and postoperative NSC scans to BCP scans suggests that NSC, particularly sagittal NSC, may affect the early development of certain white matter tracts. We also cannot rule out the possibility that some of these effects may be attributed to the surgical intervention itself; however, we aimed to provide an adequate period between surgery and postoperative scan to minimize this likelihood. Microstructural development of the corpus body and genu and inferior and superior longitudinal fasciculi appears to consistently lag behind the development observed in the BCP cohort (Tables 1, 2, 3, and 4). In BCP scans, FA values increase while MD and RD values decrease in each of these regions. In both the sagittal sub-cohort and complete NSC cohort, no significant changes in FA, MD, or RD were observed in the corpus body. FA was observed to increase in the corpus genu in the complete NSC cohort, but did not change in the sagittal subset, and MD and RD did not change in the corpus genu in either of the NSC cohorts. Low FA values in the corpus callosum measured in children have been associated with emotional dysregulation states such as bipolar disorder [14, 16]. Furthermore, aberrantly low values of FA and high values of MD in the corpus body are associated with symptoms of depression in adolescents [26]. Previous work identified a trend towards emotional dysregulation in adolescents with surgically corrected unilateral coronal synostosis [27]. Disturbances in the integrity of the corpus callosum, particularly in the body and genu, may develop in the time following surgery. Static FA and MD values between scans reflect this possible dysregulation.
Aberrant development in the SLF and ILF may also contribute to neurocognitive deficits observed long term in patients with NSC. In the sagittal subset of patients, we found no significant changes in FA and MD in the SLF. In the complete NSC cohort, we similarly found no significant changes in FA, AD, and RD in the ILF. In the BCP cohort, these parameters changed in accordance with healthy expectations in the same brain regions. In children aged 7–13, better spatial working memory performance has been associated with higher FA values in the left SLF [28]. Additionally, deficits in object recognition have been associated with lower FA in the ILF in children with cerebral visual impairment [15]. Deficiencies in fixation of visual attention and visual spatial programming have been described in patients with NSC [5]. Aberrant development of the SLF and ILF could contribute to these observed deficiencies.
In both the sagittal and complete NSC cohorts, a number of white matter tracts showed changes in DTI parameters that were not observed in the BCP cohort. This includes decreased MD in the cerebellum in both patient cohorts; decreased AD in the cerebellum and cerebellar peduncle in the complete and sagittal cohorts, respectively; decreased RD in the brain stem in the complete cohort; and increased FA in the temporal radiations for the complete cohort. While these changes differ from those observed in the BCP cohort, they all occur in the direction expected based on healthy developmental trends characterized in prior work [18,19,20]. Broad increases in FA and decreases in MD, AD, and RD are expected in the first 2 years of life. While it is possible that these changes reflect a neurodevelopmental response induced by NSC or surgical correction, it seems more likely that these are normal developmental changes. Although we utilize the BCP cohort as representative of normal development, the variation inherent in this development means that this control cohort (n = 9) may not capture the complete range of normal infant developmental patterns.
Previously, DTI measurements in five children with sagittal NSC demonstrated that FA increased aberrantly in the precuneus and decreased in the cingulum after surgical intervention [9]. While the current study did not observe these same changes between pre- and postoperative scans, it is possible that the current study’s larger sample size and more stringent significance level enabled a more granular characterization of the effects of NSC on developing white matter. Prior work found that some aberrant changes in mean diffusivity may persist into adolescence in patients with surgically corrected sagittal NSC [29]. Early surgical intervention is standard practice for infants presenting with NSC with the goal of mitigating potential effects of NSC on neurodevelopment [30]. A larger and more matched control patient population is needed to definitively assess whether the presence of NSC impacts early white matter development, as well as subsequent effects of surgical correction. It is possible that the observed discrepancies between white matter development in the BCP and NSC cohorts develop during the 5–8-month window, or that surgery itself plays a role. However, given that all infants in our cohort underwent surgical intervention, we are unable to determine the direct impact that surgery had on these areas and whether the absence of surgical intervention would produce greater microstructural disturbances. Importantly, previous work has found no differences in DTI parameters between adolescents with surgically corrected NSC and age-matched controls, suggesting an important long-term benefit of surgical intervention on white matter development [9]. Still, the natural course of white matter development in these children without surgical correction is largely unknown, so even when comparing to a healthy control group, we cannot definitively conclude how white matter would have developed in the absence of surgery.
In addition to the aforementioned neurocognitive deficits associated with NSC, deficiencies in cognition, behavior, language, attention, and inhibitory control, among others, have been noted [3, 4, 6]. fMRI in sagittal NSC patients previously found altered intrinsic connectivity within the salience network and right frontoparietal networks that persists into adolescence despite surgical intervention [9]. An additional study comparing patients with corrected NSC to controls found that patients with sagittal synostosis have decreased connectivity in the parietal lobe and unilateral coronal synostosis patients have decreased connectivity in the prefrontal cortex [31], suggesting that altered connectivity may explain observed deficits. Further investigation is needed to determine the role of white matter microstructural disturbances and the effect of surgical intervention on such disturbances.
This study has several limitations. Sample size should be considered in interpreting the results (n = 12). Additionally, the small patient cohort available for direct comparison with preoperative NSC scans (n = 5) may reduce the ability to detect differences in white matter prior to surgical intervention. The lack of a control patient population for direct comparison with our postoperative patient scans also complicated the analysis of specific effects of surgical intervention on white matter development. With current analytical techniques, we are unable to directly compare images captured using different scanning protocols (i.e., NSC and BCP patient cohorts). To best overcome this, we compared changes in white matter microstructure in NSC groups to changes measured in neurotypical controls (BCP cohort) over the same time period using the same analytical methods. Future work should compare pre- and postoperative NSC scans to more comprehensive control patient populations to better understand how NSC and how subsequent surgical correction may affect developing white matter in the infant brain. Recruitment of a control patient population age matched to both the pre- and postoperative ages of NSC scans would allow for direct comparison between these groups and a more granular characterization of white matter development in the NSC patient population.
Conclusion
White matter development from 5 to 8 months in infants with NSC, particularly sagittal NSC, undergoing cranial vault remodeling appears to be decreased in certain tracts compared to neurotypical controls. It is possible that this contributes to neurocognitive deficits previously characterized in this patient population. Investigation with a more comprehensive control dataset will provide further insight into the effects of NSC on developing white matter and the specific effects of surgical correction of these cranial deformities.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Patel A, Terner J, Travieso R, Clune JE, Steinbacher D, Persing JA (2012) On Bernard Sarnat’s 100th birthday: pathology and management of craniosynostosis. J Craniofac Surg 23. https://doi.org/10.1097/SCS.0b013e318240f
Shim KW, Park EK, Kim JS, Kim YO, Kim DS (2016) Neurodevelopmental problems in non-syndromic craniosynostosis. J Korean Neurosurg Soc 59:242–246. https://doi.org/10.3340/jkns.2016.59.3.242
Becker DB, Petersen JD, Kane AA, Cradock MM, Pilgram TK, Marsh JL (2005) Speech, cognitive, and behavioral outcomes in nonsyndromic craniosynostosis. Plast Reconstr Surg 116:400–407
Da Costa AC, Anderson VA, Holmes AD, Lo P, Wray AC, Chong DK, Greensmith AL, Meara JG (2013) Longitudinal study of the neurodevelopmental characteristics of treated and untreated nonsyndromic craniosynostosis in infancy. Child’s Nervous System 29:985–995. https://doi.org/10.1007/s00381-012-2017-0
Chieffo D, Tamburrini G, Massimi L, Di Giovanni S, Giansanti C, Caldarelli M, Di Rocco C (2010) Long-term neuropsychological development in single-suture craniosynostosis treated early: clinical article. J Neurosurg Pediatr 5:232–237. https://doi.org/10.3171/2009.10.PEDS09231
Collett BR, Kapp-Simon KA, Wallace E, Cradock MM, Buono L, Speltz ML (2017) Attention and executive function in children with and without single-suture craniosynostosis. Child Neuropsychol 23:83–98. https://doi.org/10.1080/09297049.2015.1085005
Gabrick KS, Wu RT, Singh A, Persing JA, Alperovich M (2020) Radiographic severity of metopic craniosynostosis correlates with long-term neurocognitive outcomes. Plast Reconstr Surg 145:1241–1248. https://doi.org/10.1097/PRS.0000000000006746
Aldridge K, Collett BR, Wallace ER, Birgfeld C, Austin JR, Yeh R, Feil M, Kapp-Simon KA, Aylward EH, Cunningham ML, Speltz ML (2017) Structural brain differences in school-age children with and without single-suture craniosynostosis. J Neurosurg Pediatr 19:479–489. https://doi.org/10.3171/2016.9.PEDS16107
Cabrejo R, Lacadie C, Sun A, Chuang C, Yang J, Brooks E, Beckett J, Eilbott J, Gabrick K, Steinbacher D, Duncan C, Diluna M, Alperovich M, Pelphrey K, Ventola P, Constable T, Persing JA (2021) Functional network development in sagittal craniosynostosis treated with whole vault cranioplasty. J Craniofac Surg 32:1721–1726. https://doi.org/10.1097/SCS.0000000000007505
Brooks ED, Yang J, Beckett JS, Lacadie C, Scheinost D, Persing S, Zellner EG, Oosting D, Keifer C, Friedman HE, Vander WB, Jou RJ, Sun H, Gary C, Duncan CC, Constable RT, Pelphrey KA, Persing JA (2016) Normalization of brain morphology after surgery in sagittal craniosynostosis. J Neurosurg Pediatr 17:460–468. https://doi.org/10.3171/2015.7.PEDS15221
Fontana SC, Belinger S, Daniels D, Tuttle M, Camarata PJ, Andrews BT (2018) Longitudinal assessment of developmental outcomes in infants undergoing late craniosynostosis repair. J Craniofac Surg 29:25–28. https://doi.org/10.1097/SCS.0000000000004024
Patel A, Yang JF, Hashim PW, Travieso R, Terner J, Mayes LC, Kanev P, Duncan C, Jane J, Pollack I, Losee JE, Bridgett DJ, Persing JA (2014) The impact of age at surgery on long-term neuropsychological outcomes in sagittal craniosynostosis. Plast Reconstr Surg 134:608e–617e. https://doi.org/10.1097/PRS.0000000000000511
O’Donnell LJ, Westin CF (2011) An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22:185–196
Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL (2009) Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry 66:238–244. https://doi.org/10.1016/j.biopsych.2009.02.025
Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L (2012) Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Dev Med Child Neurol 54:38–43. https://doi.org/10.1111/j.1469-8749.2011.04147.x
Saxena K, Tamm L, Walley A, Simmons A, Rollins N, Chia J, Soares JC, Emslie GJ, Fan X, Huang H (2012) A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J Child Adolesc Psychopharmacol 22:112–119. https://doi.org/10.1089/cap.2011.0063
Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. https://doi.org/10.3389/fnins.2013.00031
Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, Van Zijl PCM, Mori S (2006) Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29:493–504. https://doi.org/10.1016/J.NEUROIMAGE.2005.08.017
McGraw P, Liang L, Provenzale JM (2012) Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. Am J Roentgenol 179:1515–1522. https://doi.org/10.2214/AJR.179.6.1791515
Schneider JFL, Il’yasov KA, Hennig J, Martin E, (2004) Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology 46:258–266. https://doi.org/10.1007/S00234-003-1154-2/FIGURES/3
Mukherjee P, McKinstry RC (2006) Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am 16:19–43. https://doi.org/10.1016/J.NIC.2005.11.004
Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H (2019) Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage 185:836–850. https://doi.org/10.1016/J.NEUROIMAGE.2018.04.017
de Planque CA, Gaillard L, Vrooman HA, Li B, Bron EE, van Veelen MLC, Mathijssen IMJ, Dremmen MHG (2022) A diffusion tensor imaging analysis of frontal lobe white matter microstructure in trigonocephaly patients. Pediatr Neurol 131:42–48. https://doi.org/10.1016/j.pediatrneurol.2022.04.003
Florisson JMG, Dudink J, Koning IV, Hop WCJ, Van Veelen MLC, Mathijssen IMJ, Lequin MH (2011) Assessment of white matter microstructural integrity in children with syndromic craniosynostosis: a diffusion-tensor imaging study. Radiology 261:534–541. https://doi.org/10.1148/radiol.11101024
Rijken BFM, Leemans A, Lucas Y, Van Montfort K, Mathijssen IMJ, Lequin MH (2015) Diffusion tensor imaging and fiber tractography in children with craniosynostosis syndromes. Am J Neuroradiol 36:1558–1564. https://doi.org/10.3174/ajnr.A4301
Aghajani M, Veer IM, Van Lang NDJ, Meens PHF, Van Den Bulk BG, Rombouts SARB, Vermeiren RRJM, Van Der Wee NJ (2014) Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med 44:2287–2298. https://doi.org/10.1017/S0033291713003000
Wu RT, Yang JF, Zucconi W, Lacadie C, Swallow MS, Sun AH, Eilbott J, Mayes LC, Steinbacher DM, Pelphrey K, Persing JA (2019) Frustration and emotional regulation in nonsyndromic craniosynostosis: a functional magnetic resonance imaging study. Plast Reconstr Surg 144:1371–1383. https://doi.org/10.1097/PRS.0000000000005850
Vestergaard M, Madsen KS, Baaré WFC, Skimminge A, Rye Ejersbo L, Ramsøy TZ, Gerlach C, Paulson OB, Jernigan TL (2011) White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci 23:2135–2146
Beckett JS, Brooks ED, Lacadie C, Vander WB, Jou RJ, Steinbacher DM, Constable RT, Pelphrey KA, Persing JA (2014) Altered brain connectivity in sagittal craniosynostosis: laboratory investigation. J Neurosurg Pediatr 13:690–698. https://doi.org/10.3171/2014.3.PEDS13516
Brooks ED, Beckett JS, Yang J, Timberlake AT, Sun AH, Chuang C, Persing JA (2018) The etiology of neuronal development in craniosynostosis: a working hypothesis. J Craniofac Surg 29:49–55. https://doi.org/10.1097/SCS.0000000000004040
Sun AH, Eilbott J, Chuang C, Yang JF, Brooks ED, Beckett J, Steinbacher DM, Pelphrey K, Persing JA (2019) An investigation of brain functional connectivity by form of craniosynostosis. J Craniofac Surg 30:1719–1723. https://doi.org/10.1097/SCS.0000000000005537
Funding
Dr. Alperovich receives funding from CTSA Grant Number KL2 TR001862 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH) and consults for Johnson & Johnson and LifeNet Health.
Author information
Authors and Affiliations
Contributions
M.N.A. and M.A. conceived the experiment. M.N.A., K.G.H., J.M.H.I., and N.P. performed patient recruitment and data collection. J.M. planned the analysis with C.L. and with guidance from M.A., M.N.A., and J.A.P. C.L. performed the analysis. J.M. drafted the manuscript with critical input and revision from all authors (M.N.A., C.L., K.G.H., J.M.H.I., N.P., J.A.P., M.A.). M.A. supervised and directed the study.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Yale Institutional Review Board (HIC # 2000030969).
Consent to participate
Informed consent was obtained from legal guardians for all individual participants included in this study.
Competing interests
The authors declare no competing interests.
Disclaimer
The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Research reported in this publication was also supported by the Richard K. Gershon Endowed Medical Student Research Fellowship and Yale School of Medicine Fellowship for Medical Student Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of Richard K. Gershon Endowed Medical Student Research Fellowship and Yale School of Medicine Fellowship for Medical Student Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moscarelli, J., Almeida, M.N., Lacadie, C. et al. A diffusion tensor imaging comparison of white matter development in nonsyndromic craniosynostosis to neurotypical infants. Childs Nerv Syst 40, 1477–1487 (2024). https://doi.org/10.1007/s00381-023-06262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06262-y