Abstract
Purpose
Posthaemorrhagic ventricular dilatation in preterm infants is primarily treated using temporising measures, of which the placement of a ventricular access device (VAD) is one option. Permanent shunt dependency rates are high, though vary widely. In order to improve the treatment burden and lower shunt dependency rates, we implemented several changes over the years. One of these changes involves the setting of the surgery from general anaesthesia in the OR to local anaesthesia in bed at the neonatal intensive care unit (NICU), which may seem counterintuitive to many. In this article, we describe our surgical technique and present the results of this regimen and compare it to our previous techniques.
Methods
Retrospective study of a consecutive series of 37 neonates with posthaemorrhagic ventricular dilatation (PHVD) treated using a VAD, with a cohort I (n = 13) treated from 2004 to 2008 under general anaesthesia in the OR, cohort II (n = 11) treated from 2009 to 2013 under general anaesthesia in the NICU and cohort III (n = 13) treated from December 2013 to December 2017 under local anaesthesia on the NICU.
Results
The overall infection rate was 14%; the VAD revision rate was 22% and did not differ significantly between the cohorts. Procedures under local anaesthesia never required conversion to general anaesthesia and were well tolerated. After an average of 33 tapping days, 38% of the neonates received a permanent ventriculoperitoneal (VP) shunt. The permanent VP shunt rate was 9% with VAD placement under local anaesthesia and 52% when performed under general anaesthesia (p = 0.02).
Conclusion
Bedside placement of VADs for PHVD under local anaesthesia in neonates is a low-risk, well-tolerated procedure that results in at least equal results to surgery performed under general anaesthesia and/or performed in an OR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germinal matrix/intraventricular haemorrhage (IVH) is the most common intracranial haemorrhage among preterm infants and tends “to occur in the sickest preterm infants” [15]. A complication of intraventricular haemorrhage is the occurrence of posthaemorrhagic ventricular dilatation (PHVD) that will often result in hydrocephalus. Approximately one-third to half of the preterm infants with IVH develop PHVD [2, 3, 5]. Furthermore, there is no consensus on when to treat PHVD [3], and the optimum PHVD treatment remains unknown but may consist of temporising measures such as acetazolamide and furosemide [15], intraventricular fibrinolytic therapy [22], intermittent lumbar punctures, external ventricular drain (EVD), ventriculo-subgaleal shunting (VSGS), ventricular access devices (VAD) for intermittent CSF taps, neuroendoscopic lavage [4, 6] and definitive measures such as ventriculo-peritoneal shunting (VPS) [12]. In a significant number of cases, temporising measures still led to persisting shunt dependency, although shunt dependency rates vary widely (5–95%), with the majority over 70% [1, 2, 4, 6, 10, 11, 13, 14, 17, 19,20,21, 24].
All surgical procedures are performed under general anaesthesia and in an OR under optimised conditions to prevent complications (e.g., infections and malpositioning of catheters in ventricles increasing the risk of new intraparenchymatous haemorrhage), while the procedures themselves are relatively simple and straightforward.
In this article, we describe our alternative surgical VAD placement procedure under local anaesthesia in neonates in an incubator at the neonatal intensive care unit (NICU) and report our initial results in comparison to our previous protocols.
Methods
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The study has been approved by the institutional review board CMO Arnhem-Nijmegen (IRB). The IRB waived informed consent due to the nature of the investigations.
This is a retrospective study of all prematurely born neonates with PHVD treated with a neonatal reservoir and CSF tapping from January 2004 until December 2017 at the Radboud University Medical Center in Nijmegen, the Netherlands. From 2004 to 2009, all procedures were carried out under general anaesthesia and in the OR (cohort I). From 2009 onwards, all procedures have been carried out as a bedside procedure in the NICU, in the beginning (until November 2013) still in general anaesthesia (cohort II) and from December 2013 in local anaesthesia (cohort III).
The Neonatological Department and Paediatric Neurosurgical Department serve a total of approximately 3 million people. Annually, approximately 160 children with a maximum gestational age of 32 weeks are admitted to our neonatal intensive care unit, of whom 17% will develop an IVH, and only 14% of which will develop PHVD and come to the attention of the paediatric neurosurgeon.
In this study, PHVD is defined as a progressive ventricular enlargement exceeding the 97th percentile towards the p97 + 4 mm line [9]. Patients with a PHVD diagnosis will receive a minimum of two lumbar punctures with CSF taps, aiming for the withdrawal of 10 ml/kg. Only if the ventricular width continues to increase thereafter, the paediatric neurosurgeon is asked to place a neonatal reservoir. The reservoir is also placed in the absence of clinical symptoms and is preferably placed before symptoms of increased intracranial pressure arise.
Near-term and full-term newborns with posthaemorrhagic hydrocephalus for whom a definitive shunting was contra-indicated (for various reasons) received the same treatment as premature neonates with PHVD and were also included in this study.
For statistical analysis, the Fisher’s exact and chi-square tests were used with a P < 0.05 considered significant.
Surgical technique
VAD placement in general anaesthesia and in the OR is a simple and straightforward procedure that does not require extensive explanation. We will only discuss in detail the technique of bedside placement of a VAD on the NICU under local anaesthesia, since this is a new technique.
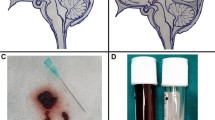
The neonate is placed crosswise in the incubator at the NICU, and both sides of the roof of the incubator are taken down. The neonate is not sober or otherwise prepared for surgery. The neonate is then orally given some glucose water using a syringe and is then swaddled using a baby’s comforter and held by a neonatal nurse (Fig. 1). If necessary, a minimal amount of hair is shaven at the level of the fontanel. Typically, < 1 ml of local anaesthesia is provided at the incision site (lidocaine 0.5% with epinephrine 1:400.000). Surgery is always performed by an experienced paediatric neurosurgeon and assisted by a scrub nurse from the neurosurgical OR, with no anaesthesiological assistance. A solution of vancomycin (500 mg in 250 ml NaCl 0.9%) is used for topical use, and the wetting of surgical gauzes and drenching of the implant are performed in accordance with our shunt infection prevention protocol [18]. The lateralisation of the incision is planned dependent on the ventricular size and intraventricular clot formation, so that the catheter is placed in the largest ventricle and/or the ventricle with the smallest blood clot. After skin disinfection using Betadine, a single small sterile drape with a central hole is placed over the incision line. A 1-cm skin incision is made 1–1.5 cm across next to the midline at the level of the coronary suture in the open fontanel, after which a small subgaleal pocket is created just over the frontal bone oblique to the incision using scissors (Fig. 1). Bipolar coagulation is applied to the periosteum/dura, after which this is punctured using the same surgical knife (size 15). The pia mater is coagulated, and then a ventriculostomy is performed using a Dandy cannula. The Dandy cannula is then removed, and a 35-mm or 45-mm (preoperatively chosen on ultrasound measurement) one-piece neonatal reservoir (Medtronic®, Fridley, Minnesota, USA) is introduced into the ventricle and the reservoir is placed in the subgaleal pocket and on the frontal bone (Fig. 1). Wound closure is performed by single-layer rapidly resorbable 4-0 suture and covered by a transparent wound dressing.
Postoperatively, ultrasound is performed on a daily basis, and reservoir taps are performed as per institutional tapping protocol by the neonatologist, initially twice daily. The paediatric neurosurgeon is not involved anymore, unless the hydrocephalus persists after a prolonged period following CSF tapping, and tapping cannot be reduced or the VAD gets obstructed. A permanent CSF drainage is only considered if a weight of 2.5 kg is reached and/or a minimum of 2 months of CSF tapping exists with no tendency to improve. The indication to permanent shunting is therefore taken by the neonatologist rather than the neurosurgeon.
Results
The baseline characteristics of the three cohorts are summarized in Table 1. A total of 35 neonates with PHVD have been treated with a VAD placement, while 1 child born at 40 weeks of gestation with an intraventricular haemorrhage and 1 new born child with an acute subdural hematoma and concomitant hydrocephalus received an identical treatment regimen as with PHVD.
The three cohorts consisted of 13, 11 and 13 patients. Cohorts I and II were very similar, whereas in cohort III, patients tended to be a little older (due to 2 cases that were not prematurely born) and thus also a little heavier (Table 1). Surgical characteristics were very similar between the groups.
The overall infection rate of the VADs was 14% and actually lower if surgery was performed in bed on the NICU than in the OR, 8% versus 23% (n.s.). Surgical revision because of VAD dysfunction/obstruction was required in 22% of cases, 15% if performed in the OR and 25% if performed in the NICU in the incubator (n.s.).
In all cases of local anaesthesia (cohort III), the procedure could be conducted without additional pain treatment or conversion to sedation or general anaesthesia and was well tolerated by the neonates. The average procedural time was 6 min. There were no intraoperative complications. The three obstructed reservoirs in this cohort were replaced by a new reservoir in a similar procedure under local anaesthesia. Five neonates (14%) died while having a VAD and receiving daily CSF tapping: one as a result of a status epilepticus and one due to necrotising enterocolitis, while in three cases treatment was discontinued because of a dismal overall prognosis. In none of these five cases, death was related to hydrocephalus, complications of surgery or tapping the reservoirs.
Overall, 38% of surviving PHVD patients required a VPS. There is a significant difference between the three cohorts, with only 1 of the 11 surviving patients in cohort III requiring a VPS (p = 0.044). When comparing procedures in general anaesthesia (cohort I and II) with procedures in local anaesthesia (cohort III), this difference is significant at p = 0.02.
Discussion
In our department, we prefer to place ventricular access devices (subcutaneous neonatal CSF reservoirs) for PHVD, perform frequent and prolonged CSF tapping and ultimately convert to a VPS if necessary. In order to decrease our complication rate and the shunt dependency rate, we have altered our protocols and procedures several times over a period of 14 years. First, in 2008, we altered the setting of this procedure from the OR and being performed by a general neurosurgeon or resident to a procedure performed in the incubator at the NICU by a dedicated paediatric neurosurgeon, though still under general anaesthesia by a paediatric anaesthesiologist. In 2010, we improved the protocol for tapping of the VAD in order to decrease the infection rate. Over time, we came to the conclusion that for a surgical procedure this simple and rapid (being 5–6 min in length), general anaesthesia with intubation of the patient is even more invasive, risky and a greater burden on the patient than the surgical procedure itself. In close collaboration with the neonatologists, we decided to attempt performing the procedure under local anaesthesia and started with this new protocol in December 2013 and have continued doing so. In our consecutive series, we have demonstrated that, with the described technique of VAD placement in local anaesthesia, the procedure can just as safely and easily be performed as when performed in general anaesthesia and/or in the OR. The malpositioning rate of the catheter and obstruction rate of the catheter did not increase, while occluded reservoirs could just as easily be replaced by new ones in a similar rapid procedure under local anaesthesia. Also, the infection rate was not higher than in other series in which surgery was performed in the OR [16, 20, 21]. However, the three cohorts are small and do not allow definitive conclusions.
We determined that the procedure in local anaesthesia is well tolerated by this vulnerable group of patients. Our neonatologists absolutely prefer this procedure using local anaesthesia over general anaesthesia, especially because anaesthesia-induced hypotension is prevented and hypoxia associated with intubation and extubation does not occur, while a neonate on the ventilator due to lung problems can just as easily be operated on in the same manner. Furthermore, the risk associated with patient transport through the hospital to the OR is avoided. Moreover, the conversion of local to general anaesthesia was never required.
The neurosurgical literature is very sparse of reports on procedures in local anaesthesia or bedside procedures in this group of patients. Januschek et al. and Zucchelli et al. have described the bedside placement of external ventricular reservoirs in the NICU with good results in these two small series (technical note and clinical series) and without observing increased infection rates. Zucchelli et al. [25] performed these procedures in general anaesthesia, while Januschek et al. [8] do not mention the type of anaesthesia. In both series, the procedure was performed as a temporizing measure for ultra and very low birth weight neonates (< 1000 g) while planning VPS placement (if indicated) once neonates reached a weight of > 1000 g. Both authors conclude that EVD placement can be safely performed in the NICU, avoiding transports through the hospital and getting the neonate outside the incubator.
Hagander et al. [7] already demonstrated that even major abdominal surgery can be relatively safely performed under local anaesthesia in premature infants and represents an established option, especially in developing countries where general anaesthesia remains accompanied by significant mortality.
VP shunting rate
With our surgical treatment protocol, we achieved a very low definitive shunt conversion rate of 9%, which is significantly lower than our previous treatment regimen and significantly lower than the majority of published studies. However, the factor that contributed most to our low shunt conversion rate remains unknown. Numerous studies on the surgical treatment of PHVD differ widely in the type of surgery performed and also in the timing of intervention. As such, there is no consensus regarding whether to start intervention early at the first sign of PHVD (97th percentile), later (97th percentile + 4 mm or more) or when the signs and symptoms of raised intracranial pressure occur [3]. However, we tend to intervene early and believe that this contributes significantly to a low shunt conversion rate. Some studies have shown that early intervention may lead to a lower permanent shunt rate and/or better neurological outcomes, whereas studies with late interventions had very high permanent shunt rates [2, 5, 14, 23]. In a recent prospective multicentre cohort study from the Hydrocephalus Clinical Research Network, permanent shunt rates after VSGS and VAD were 63.5% and 74%, respectively [20]. However, patients were only considered for treatment if FOHR > 0.55 and they were clinically symptomatic (of at least two of bradycardia, split sutures or bulging fontanel). In our opinion, this represents very late surgery, which contrasts the majority of neonates with few or no symptoms in our study.
Also, the period of reservoir tapping, the daily number of taps, and the amount of CSF drawn from a reservoir or alternative temporising measures before permanent shunt is indicated vary widely. Some wait until the child is 2000 g or 2500 g, while others limit the number of punctures or prefer a permanent treatment to allow discharge from the hospital. In our hospital, we tend to prolong hospitalisation to provide the child a chance upon recovering from PHVD and will continue reservoir tapping once or twice daily for up to 2 months or longer, especially if an initial trend in the reduction of tapping frequency is observed. Willis et al. [24] treated 32 premature infants, of whom 17 directly received a VP shunt, while 15 received a tapping reservoir. Of the latter, hydrocephalus was halted in only 2, and 13 received a VP shunt afterward. They used a prolonged tapping period similar to ours; nevertheless, a high permanent shunt rate was observed. Information on the timing of the intervention is not available in this study, and this study is therefore not comparable to ours. In their series of 95 patients, Limbrick et al. [10] observed shunt rates of 75% for VAD and 67% for VSGS. The ventricular size is not mentioned, though a temporising measure is only taken in neonates who exhibit clinical signs of raised ICP, suggesting very late first interventions. Bock et al. [1] also tap VADs for a prolonged period of up to three times a day; however, they tap 2–5 ml—much lower than our 10 ml/kg. Their shunting rate is 95%. Notably, Tian et al. [17] presented a series of VADs with a 0% infection rate, a very low complication rate and a tapping protocol quite similar to ours; nevertheless, they observed a shunt conversion rate of 70%. We were unable to identify factors that can explain the differences in outcome compared with our series. The primary difference is local anaesthesia and bedside surgery in contrast to general anaesthesia and surgery in the OR. However, we find it very unlikely that this difference can fully explain the difference in outcomes with regard to shunt conversion rates.
Our cohort of patients is unselective, but also very small, which may have attributed significant bias. We have established that a simpler and less elaborate procedure of VAD placement can be just as safe and effective. The observed effect of a lower shunting rate is not explained for but remarkable and should trigger further evaluation in a larger series.
Conclusion
Bedside placement of VADs under local anaesthesia in neonates is a low-risk, well-tolerated procedure. Alongside an early intervention and a prolonged and intensified VAD tapping regimen, this leads to very good results with very low definitive CSF shunting and a low complication rate in our small series. Further prospective studies are needed to examine whether these favourable results can be maintained in larger and multicentre patient cohorts.
References
Bock HC, Feldmann J, Ludwig HC (2018) Early surgical management and long-term surgical outcome for intraventricular hemorrhage-related posthemorrhagic hydrocephalus in shunt-treated premature infants. J Neurosurg Pediatr 22:61–67. https://doi.org/10.3171/2018.1.PEDS17537
Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L (2008) Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr 152:648–654. https://doi.org/10.1016/j.jpeds.2007.10.005
Brouwer AJ, Brouwer MJ, Groenendaal F, Benders MJ, Whitelaw A, de Vries LS (2012) European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed 97:F50–F55. https://doi.org/10.1136/adc.2010.207837
d’Arcangues C, Schulz M, Buhrer C, Thome U, Krause M, Thomale UW (2018) Extended experience with neuroendoscopic lavage for posthemorrhagic hydrocephalus in neonates. World Neurosurg 116:e217–e224. https://doi.org/10.1016/j.wneu.2018.04.169
de Vries LS, Liem KD, van Dijk K, Smit BJ, Sie L, Rademaker KJ, Gavilanes AW, Dutch Working Group of Neonatal N (2002) Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in The Netherlands. Acta Paediatr 91:212–217
Gaderer C, Schaumann A, Schulz M, Thomale UW (2018) Neuroendoscopic lavage for the treatment of CSF infection with hydrocephalus in children. Childs Nerv Syst 34:1893–1903. https://doi.org/10.1007/s00381-018-3894-7
Hagander L, Kabir M, Chowdhury MZ, Gunnarsdottir A, Habib MG, Banu T (2015) Major neonatal surgery under local anesthesia: a cohort study from Bangladesh. World J Surg 39:953–960. https://doi.org/10.1007/s00268-014-2895-2
Januschek E, Machado LS, Steinthal B, Ulrich PT (2011) Posthemorrhagic hydrocephalus in very low birth weight infants--a new gentle surgical technique for external ventricular drainage. Childs Nerv Syst 27:991–994. https://doi.org/10.1007/s00381-011-1413-1
Levene MI (1981) Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56:900–904
Limbrick DD Jr, Mathur A, Johnston JM, Munro R, Sagar J, Inder T, Park TS, Leonard JL, Smyth MD (2010) Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr 6:224–230. https://doi.org/10.3171/2010.5.PEDS1010
Mauer UM, Unterreithmeir L, Jahn A, Wagner W, Kunz U, Schulz C (2013) A survey on current practice in the neurosurgical management of preterm infants with posthemorrhagic hydrocephalus in Germany. J Neurol Surg A Cent Eur Neurosurg 74:82–86. https://doi.org/10.1055/s-0032-1320023
Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr, Rogido M, Mitchell L, Flannery AM, Pediatric Hydrocephalus Systematic R, Evidence-Based Guidelines Task F (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr 14(Suppl 1):8–23. https://doi.org/10.3171/2014.7.PEDS14322
Richard E, Cinalli G, Assis D, Pierre-Kahn A, Lacaze-Masmonteil T (2001) Treatment of post-haemorrhage ventricular dilatation with an Ommaya’s reservoir: management and outcome of 64 preterm infants. Childs Nerv Syst 17:334–340
Riva-Cambrin J, Shannon CN, Holubkov R, Whitehead WE, Kulkarni AV, Drake J, Simon TD, Browd SR, Kestle JR, Wellons JC 3rd, Hydrocephalus Clinical Research N (2012) Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. J Neurosurg Pediatr 9:473–481. https://doi.org/10.3171/2012.1.PEDS11292
Robinson S (2012) Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr 9:242–258. https://doi.org/10.3171/2011.12.PEDS11136
Spader HS, Hertzler DA, Kestle JR, Riva-Cambrin J (2015) Risk factors for infection and the effect of an institutional shunt protocol on the incidence of ventricular access device infections in preterm infants. J Neurosurg Pediatr 15:156–160. https://doi.org/10.3171/2014.9.PEDS14215
Tian AG, Hintz SR, Cohen RS, Edwards MS (2012) Ventricular access devices are safe and effective in the treatment of posthemorrhagic ventricular dilatation prior to shunt placement. Pediatr Neurosurg 48:13–20. https://doi.org/10.1159/000337876
van Lindert EJ, Bilsen MV, Flier MV, Kolwijck E, Delye H, Oever JT (2018) Topical vancomycin reduces the cerebrospinal fluid shunt infection rate: a retrospective cohort study. PLoS One 13:e0190249. https://doi.org/10.1371/journal.pone.0190249
Wang JY, Amin AG, Jallo GI, Ahn ES (2014) Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr 14:447–454. https://doi.org/10.3171/2014.7.PEDS13552
Wellons JC 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni AV, Limbrick DD Jr, Whitehead W, Browd S, Rozzelle C, Simon TD, Tamber MS, Oakes WJ, Drake J, Luerssen TG, Kestle J, Hydrocephalus Clinical Research N (2017) Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J Neurosurg Pediatr 20:19–29. https://doi.org/10.3171/2017.1.PEDS16496
Wellons JC, Shannon CN, Kulkarni AV, Simon TD, Riva-Cambrin J, Whitehead WE, Oakes WJ, Drake JM, Luerssen TG, Walker ML, Kestle JR, Hydrocephalus Clinical Research N (2009) A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr 4:50–55. https://doi.org/10.3171/2009.2.PEDS08400
Whitelaw A, Evans D, Carter M, Thoresen M, Wroblewska J, Mandera M, Swietlinski J, Simpson J, Hajivassiliou C, Hunt LP, Pople I (2007) Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics 119:e1071–e1078. https://doi.org/10.1542/peds.2006-2841
Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, Hunt L, Carter M, Pople I (2010) Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125:e852–e858. https://doi.org/10.1542/peds.2009-1960
Willis B, Javalkar V, Vannemreddy P, Caldito G, Matsuyama J, Guthikonda B, Bollam P, Nanda A (2009) Ventricular reservoirs and ventriculoperitoneal shunts for premature infants with posthemorrhagic hydrocephalus: an institutional experience. J Neurosurg Pediatr 3:94–100. https://doi.org/10.3171/2008.11.PEDS0827
Zucchelli M, Lefosse M, Corvaglia L, Martini S, Sandri F, Soffritti S, Ancora G, Mammoliti P, Gargano G, Galassi E (2016) Introduction of percutaneous-tunneled transfontanellar external ventricular drainage in the management of hydrocephalus in extremely low-birth-weight infants. J Neurosurg Pediatr 18:1–6. https://doi.org/10.3171/2016.1.PEDS15563
Acknowledgements
We are indebted to Maartje Kunen, medical illustrator of Medical Visuals, Arnhem, Netherlands, for the production of the illustration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The study has been approved by the institutional review board CMO Arnhem-Nijmegen (IRB). The IRB waived informed consent due to the nature of the investigations.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Lindert, E.J., Liem, K.D., Geerlings, M. et al. Bedside placement of ventricular access devices under local anaesthesia in neonates with posthaemorrhagic hydrocephalus: preliminary experience. Childs Nerv Syst 35, 2307–2312 (2019). https://doi.org/10.1007/s00381-019-04361-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04361-3