Abstract
Purpose
Hydrocephalus (HC) has a multifactorial and complex picture of pathophysiology due to aetiology, age at and duration since onset. We have previously identified distinctions in markers of cell death associated with different aetiologies. Here, we examined cerebrospinal fluid (CSF) from human HC neonates for cytokines to identify further distinguishing features of different aetiologies.
Methods
CSF was collected during routine lumbar puncture or ventricular tap from neonates with hydrocephalus, or with no neurological condition (normal controls). Total protein, Fas receptor, Fas ligand, stem cell factor (SCF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), insulin growth factor-1 (IGF-1), tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were measured and compared between 8 unaffected and 28 HC neonatal CSF samples.
Results
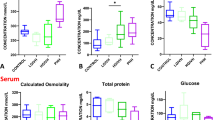
Total protein was significantly (P < 0.05) raised in late-onset hydrocephalus (LOH). Fas receptor was raised (P < 0.05) in post-haemorrhagic hydrocephalus (PHH) and spina bifida with hydrocephalus (SB/HC), but no difference in Fas ligand was found. SCF was raised (P < 0.05) in SB/HC. HGF was found in all HC and was increased (P < 0.01) in PHH. Increased VEGF was found in PHH (P < 0.01) and SB/HC (P < 0.05). Variable levels of IL-6, TNF-α and IGF-1 were found in all HC groups compared with none in normal.
Conclusions
LOH was unusual with significantly raised total protein indicating an inflammatory state. Increased Fas receptor, VEGF, IGF-1 and HGF suggest anti-apoptotic and repair mechanism activation. By contrast, elevated TNF-α and IL-6 indicate inflammatory processes in these neonatal brains. Taken with our previous study, these data indicate that different pathophysiology, inflammation and repair are occurring in HC of different aetiologies and that additional treatment strategies may benefit these infants in addition to fluid diversion.



Similar content being viewed by others
References
Allan SM, Parker LC, Collins B, Davies R, Luheshi GN, Rothwell NJ (2000) Cortical cell death induced by IL-1 is mediated via actions in the hypothalamus of the rat. Proc Natl Acad Sci U S A 97:5580–5585
Andre R, Pinteaux E, Kimber I, Rothwell NJ (2005) Differential actions of IL-1 alpha and IL-1 beta in glial cells share common IL-1 signalling pathways. Neuroreport 16:153–157
Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F (1995) IGF1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14:717–730
Benveniste EN (1998) Cytokine actions in the central nervous system. Cytokine Growth Factor Rev 9:259–275
Berse B, Brown LF, Vandewater L, Dvorak HF, Senger DR (1992) Vascular-permeability factor (vascular endothelial growth-factor) gene is expressed differentially in normal-tissues, macrophages, and tumors. Mol Biol Cell 3:211–220
Brett FM, Mizisin AP, Powell HC, Campbell IL (1995) Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J Neuropathol Exp Neurol 54:766–775
Buddensiek J, Dressel A, Kowalski M, Storch A, Sabolek M (2009) Adult cerebrospinal fluid inhibits neurogenesis but facilitates gliogenesis from fetal rat neural stem cells. J Neurosci Res 87:3054–3066
Buddensiek J, Dressel A, Kowalski M, Runge U, Schroeder H, Hermann A, Kirsch M, Storch A, Sabolek M (2010) Cerebrospinal fluid promotes survival and astroglial differentiation of adult human neural progenitor cells but inhibits proliferation and neuronal differentiation. BMC Neurosci 11:48
Bunn RC, King WD, Winkler MK, Fowlkes JL (2005) Early developmental changes in IGF-I, IGF-II, IGF binding protein-1, and IGF binding protein-3 concentration in the cerebrospinal fluid of children. Pediatr Res 58:89–93
Cheng HJ, Flanagan JG (1994) Transmembrane kit ligand cleavage does not require a signal in the cytoplasmic domain and occurs at a site dependent on spacing from the membrane. Mol Biol Cell 5:943–953
Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD (1994) Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263:1759–1762
Czosnyka M, Czosnyka Z, Schmidt EA, Momjian S (2003) Cerebrospinal fluid production. J Neurosurg 99:206–207
Dang I, Nelson JK, DeVries GH (2005) c-Kit receptor expression in normal human Schwann cells and Schwann cell lines derived from neurofibromatosis type 1 tumors. J Neurosci Res 82:465–471
Di Renzo MF, Bertolotto A, Olivero M, Putzolu P, Crepaldi T, Schiffer D, Pagni CA, Comoglio PM (1993) Selective expression of the Met/HGF receptor in human central nervous system microglia. Oncogene 8:219–222
Dinarello CA (1998) Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol 16:457–499
Dougherty JM, Roth RM (1986) Cerebral spinal fluid. Emerg Med Clin North Am 4:281–297
Dziegielewska KM, Knott GW, Saunders NR (2000) The nature and composition of the internal environment of the developing brain. Cell Mol Neurobiol 20:41–56
Erlandsson A, Larsson J, Forsberg-Nilsson K (2004) Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp Cell Res 301:201–210
Eskandari R, Harris CA, McAllister JP 2nd (2011) Reactive astrocytosis in feline neonatal hydrocephalus: acute, chronic, and shunt-induced changes. Childs Nerv Syst 27:2067–2076
Fassbender K, Eschenfelder C, Hennerici M (1999) Fas (APO-1/CD95) in inflammatory CNS diseases: intrathecal release in bacterial meningitis. J Neuroimmunol 93:122–125
Felderhoff-Mueser U, Herold R, Hochhaus F, Koehne P, Ring-Mrozik E, Obladen M, Buhrer C (2001) Increased cerebrospinal fluid concentrations of soluble Fas (CD95/Apo-1) in hydrocephalus. Arch Dis Child 84:369–372
Ferrara N (2000) Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 55:15–35
Fishman RA (1992) Cerebrospinal fluid in diseases of the nervous system. Saunders, Philadelphia
Fukumizu M, Takashima S, Becker LE (1995) Neonatal posthemorrhagic hydrocephalus: neuropathologic and immunohistochemical studies. Pediatr Neurol 13:230–234
Gato A, Moro JA, Alonso MI, Bueno D, De La Mano A, Martin C (2005) Embryonic cerebrospinal fluid regulates neuroepithelial survival, proliferation, and neurogenesis in chick embryos. Anat Rec A: Discov Mol Cell Evol Biol 284A:475–484
Gato A, Desmond ME (2009) Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol 327:263–272
Gerber J, Tumani H, Kolenda H, Nau R (1998) Lumbar and ventricular CSF protein, leukocytes, and lactate in suspected bacterial CNS infections. Neurology 51:1710–1714
Gibson RM, Rothwell NJ, Le Feuvre RA (2004) CNS injury: the role of the cytokine IL-1. Vet J 168:230–237
Gopinath G, Bhatia R, Gopinath PG (1979) Ultrastructural observations in experimental hydrocephalus in the rabbit. J Neurol Sci 43:333–334
Gruol DL, Nelson TE (1997) Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol 15:307–339
Habgood MD, Knott GW, Dziegielewska KM, Saunders NR (1993) The nature of the decrease in blood-cerebrospinal fluid barrier exchange during postnatal brain development in the rat. J Physiol 468:73–83
Harris NG, Jones HC, Patel S (1994) Ventricle shunting in young H-Tx rats with inherited congenital hydrocephalus: a quantitative histological study of cortical grey matter. Childs Nerv Syst 10:293–301, discussion 301
Hughes DP, Crispe IN (1995) A naturally occurring soluble isoform of murine Fas generated by alternative splicing. J Exp Med 182:1395–1401
Hunter G, Smith HV (1960) Calcium and magnesium in human cerebrospinal fluid. Nature 186:161–162
Jin K, Mao XO, Sun Y, Xie L, Greenberg DA (2002) Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest 110:311–319
Jin KL, Mao XO, Greenberg DA (2000) Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A 97:10242–10247
John CC, Panoskaltsis-Mortari A, Opoka RO, Park GS, Orchard PJ, Jurek AM, Idro R, Byarugaba J, Boivin MJ (2008) Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg 78:198–205
Kahle PJ, Jakowec M, Teipel SJ, Hampel H, Petzinger GM, Di Monte DA, Silverberg GD, Moller HJ, Yesavage JA, Tinklenberg JR, Shooter EM, Murphy GM Jr (2000) Combined assessment of tau and neuronal thread protein in Alzheimer's disease CSF. Neurology 54:1498–1504
Kern MA, Bamborschke S, Nekic M, Schubert D, Rydin C, Lindholm D, Schirmacher P (2001) Concentrations of hepatocyte growth factor in cerebrospinal fluid under normal and different pathological conditions. Cytokine 14:170–176
Khurana RK (1996) Intracranial hypotension. Semin Neurol 16:5–10
Kirchhoff C, Stegmaier J, Bogner V, Buhmann S, Mussack T, Kreimeier U, Mutschler W, Biberthaler P (2006) Intrathecal and systemic concentration of NT-proBNP in patients with severe traumatic brain injury. J Neurotrauma 23:943–949
Krasnoselsky A, Massay MJ, DeFrances MC, Michalopoulos G, Zarnegar R, Ratner N (1994) Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. J Neurosci 14:7284–7290
Krebs V, Suely O, Okay Y, Vaz F (2004) Tumor necrosis factor-a, interleukin-1b and interleukin-6 in the cerebrospinal fluid of newborn with meningitis. Arq Neuropsiquiatr 63(1):7–13
Lai Y, Stange C, Wisniewski SR, Adelson PD, Janesko-Feldman KL, Brown DS, Kochanek PM, Clark RSB (2006) Mitochondrial heat shock protein 60 is increased in cerebrospinal fluid following pediatric traumatic brain injury. Dev Neurosci 28:336–341
Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D'Ercole AJ, Wong ET, LaMantia AS, Walsh CA (2011) The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905
Leinonen V, Menon LG, Carroll RS, Dello Iacono D, Grevet J, Jaaskelainen JE, Black PM (2011) Cerebrospinal fluid biomarkers in idiopathic normal pressure hydrocephalus. Int J Alzheimer's Dis 2011:312526
Madsen JR, Shim JW, Abazi G, Fleming L, Fernholz B, Connors S, Folkman J (2009) VEGF-A is elevated in CSF of pediatric patients undergoing surgery for hydrocephalus. 52nd Annual Meeting of the Society for Research into Hydrocephalus and Spina Bifida
Maina F, Klein R (1999) Hepatocyte growth factor, a versatile signal for developing neurons. Nat Neurosci 2:213–217
Martin-Ancel A, Garcia-Alix A, Pascual-Salcedo D, Cabanas F, Valcarce M, Quero J (1997) Interleukin-6 in the cerebrospinal fluid after perinatal asphyxia is related to early and late neurological manifestations. Pediatrics 100:789–794
Martin C, Alonso MI, Santiago C, Moro JA, De la Mano A, Carretero R, Gato A (2009) Early embryonic brain development in rats requires the trophic influence of cerebrospinal fluid. Int J Dev Neurosci 27:733–740
Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M (2001) Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J 15:1218–1220
McAllister II JP, Forsyth J, Deren KE (2009) Global neuroinflammation patterns in experimental neonatal hydrocephalus. 53rd Annual Meeting of the Society for Research into Hydrocephalus and Spina Bifida, Belfast
McAllister JP 2nd (2012) Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med 17:285–294
Milhorat TH (1972) Hydrocephalus and the cerebrospinal fluid. Williams and Wilkins Co, Baltimore
Milhorat TH, Davis DA, Hammock MK (1975) Localization of ouabin-sensitive Na-K ATPase in frog, rabbit and rat choroid plexus. Brain Res 99:170–174
Miller JM, McAllister JP 2nd (2007) Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res 4:5
Miyan JA, Zendah M, Mashayekhi F, Owen-Lynch PJ (2006) Cerebrospinal fluid supports viability and proliferation of cortical cells in vitro, mirroring in vivo development. Cerebrospinal Fluid Res 3:2
Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett 211:13–16
Nabiuni M, Rasouli J, Parivar K, Kochesfehani HM, Irian S, Miyan JA (2012) In vitro effects of fetal rat cerebrospinal fluid on viability and neuronal differentiation of PC12 cells. Fluids Barriers CNS 9:8
Nagashima H, Morio Y, Yamane K, Nanjo Y, Teshima R (2009) Tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy. Eur Spine J 18:1946–1950
Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456
Nagra G, Li J, McAllister JP 2nd, Miller J, Wagshul M, Johnston M (2008) Impaired lymphatic cerebrospinal fluid absorption in a rat model of kaolin-induced communicating hydrocephalus. Am J Physiol Regul Integr Comp Physiol 294:R1752–R1759
Nanba R, Kuroda S, Ishikawa T, Houkin K, Iwasaki Y (2004) Increased expression of hepatocyte growth factor in cerebrospinal fluid and intracranial artery in moyamoya disease. Stroke 35:2837–2842
Naureen I, Waheed KA, Rathore AW, Victor S, Mallucci C, Goodden JR, Chohan SN, Miyan JA (2013) Fingerprint changes in CSF composition associated with different aetiologies in human neonatal hydrocephalus: glial proteins associated with cell damage and loss. Fluids Barriers CNS 10:34
Nayeri F, Nilsson I, Hagberg L, Brudin L, Roberg M, Soderstrom C, Forsberg P (2000) Hepatocyte growth factor levels in cerebrospinal fluid: a comparison between acute bacterial and nonbacterial meningitis. J Infect Dis 181:2092–2094
Ozden M, Kalkan A, Demirdag K, Denk A, Kilic SS (2004) Hepatocyte growth factor (HGF) in patients with hepatitis B and meningitis. J Infect 49:229–235
Pallis C, MacIntyre I, Anstall H (1965) Some observations on magnesium in cerebrospinal fluid. J Clin Pathol 18:762–764
Parada C, Martin C, Alonso MI, Moro JA, Bueno D, Gato A (2005) Embryonic cerebrospinal fluid collaborates with the isthmic organizer to regulate mesencephalic gene expression. J Neurosci Res 82:333–345
Pattisapu JV (2001) Etiology and clinical course of hydrocephalus. Neurosurg Clin N Am 12:651–659
Reiber H (1994) Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 122:189–203
Riikonen R, Makkonen I, Vanhala R, Turpeinen U, Kuikka J, Kokki H (2006) Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev Med Child Neurol 48:751–755
Savman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H (1998) Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res 43:746–751
Schoenle EJ, Haselbacher GK, Briner J, Janzer RC, Gammeltoft S, Humbel RE, Prader A (1986) Elevated concentration of IGF II in brain tissue from an infant with macrencephaly. J Pediatr 108:737–740
Schoniger S, Kopp MDA, Schomerus C, Maronde E, Dehghani F, Meiniel A, Rodriguez M, Korf HW, Nurnberger F (2002) Effects of neuroactive substances on the activity of subcommissural organ cells in dispersed cell and explant cultures. Cell Tissue Res 307:101–114
Senger DR, Perruzzi CA, Feder J, Dvorak HF (1986) A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res 46:5629–5632
Streffer JR, Schuster M, Zipp F, Weller M (1998) Soluble CD95 (Fas/APO-1) in malignant glioma: (no) implications for CD95-based immunotherapy? J Neurooncol 40:233–235
Tarkowski E, Tullberg M, Fredman P, Wikkelso C (2003) Normal pressure hydrocephalus triggers intrathecal production of TNF-alpha. Neurobiol Aging 24:707–714
Tonnesen MG, Feng X, Clark RA (2000) Angiogenesis in wound healing. J Investig Dermatol Symp Proc 5:40–46
Torres-Aleman I, Villalba M, Nieto-Bona MP (1998) Insulin-like growth factor-I modulation of cerebellar cell populations is developmentally stage-dependent and mediated by specific intracellular pathways. Neuroscience 83:321–334
Tsuzuki N, Miyazawa T, Matsumoto K, Nakamura T, Shima K, Chigasaki H (2000) Hepatocyte growth factor reduces infarct volume after transient focal cerebral ischemia in rats. Acta Neurochir Suppl 76:311–316
Weller RO, Wisniewski H (1969) Histological and ultrastructural changes with experimental hydrocephalus in adult rabbits. Brain 92:819–828
Werther GA, Russo V, Baker N, Butler G (1998) The role of the insulin-like growth factor system in the developing brain. Horm Res 49:37–40
Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J (2011) Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 3:99ra87
Zappaterra MW, Lehtinen MK (2012) The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci 69:2863–2878
Zhang J, Williams MA, Rigamonti D (2006) Genetics of human hydrocephalus. J Neurol 253:1255–1266
Zhang SC, Fedoroff S (1997) Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J Neurosci Res 47:1–15
Zhang W, Yi MJ, Chen X, Cole F, Krauss RS, Kang JS (2006) Cortical thinning and hydrocephalus in mice lacking the immunoglobulin superfamily member CDO. Mol Cell Biol 26:3764–3772
Zipp F, Otzelberger K, Dichgans J, Martin R, Weller M (1998) Serum CD95 of relapsing remitting multiple sclerosis patients protects from CD95-mediated apoptosis. J Neuroimmunol 86:151–154
Acknowledgments
Financial support was provided by The Charles Wolfson Charitable Trust (JAM). Miss Naureen was supported through a British Council INSPIRE grant (SP055) to The University of Manchester, UK and COMSATS Institute for Information Technology, Islamabad, Pakistan that also supported the work carried out in Islamabad and Lahore. We thank Dr. Nadeem Malik (neurosurgeon) and the Neonatology registrars at the Children’s Hospital Lahore for CSF collections in Pakistan; Sasha Burn, Dawn Williams, Clare Jennings, Anna Hendrickson and Jennifer Hill for CSF collections in the UK; Professor M. Akhtar, FRS and Dr Naeem of the School of Biological Sciences, University of Punjab, Lahore for access to laboratory facilities for sample preparation and lyophilisation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naureen, I., Waheed, K.A.I., Rathore, A.W. et al. Fingerprint changes in CSF composition associated with different aetiologies in human neonatal hydrocephalus: inflammatory cytokines. Childs Nerv Syst 30, 1155–1164 (2014). https://doi.org/10.1007/s00381-014-2415-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2415-6