Abstract
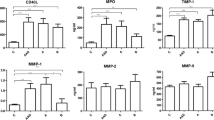
Bicuspid aortic valve (BAV) exhibits a clinical incline toward aortopathy, in which aberrant tensile and shear stress generated by BAV can induce differential expression of matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs). Whether stenotic BAV, which exhibits additional eccentric high-velocity flow jet upon ascending aorta and further worsens circumferential systolic wall shear stress than BAV with echocardiographically normal aortic valve, can lead to unique plasma MMP/TIMP patterns is still unknown. According to their valvulopathy and aortic dilatation status, 93 BAV patients were included in the present study. Group A (n = 37) and B (n = 28) comprised severely stenotic patients with or without ascending aorta dilatation; Group C (n = 12) and D (n = 16) comprised echocardiographically normal BAV patients with or without ascending aorta dilatation. Plasma MMP/TIMP levels (MMP-1, -2, -3, -8, -9, -10, -13 and TIMP-1, -2, -4) were determined via a multiplex ELISA detection system in a single procedure. Among patients with isolated severe aortic stenosis, plasma levels of MMP-2 and -9 were significantly elevated when ascending aortic dilatation was present (p = 0.001 and p = 0.002, respectively). MMP-2, however, remained as the single elevated plasma component among echocardiographically normal BAV patients with dilated ascending aorta (p = 0.027). Multivariate analysis revealed that MMP-2 and MMP-9 could both serve as independent risk factor for aortic dilatation in the case of isolated severe stenosis (p = 0.003 and p = 0.001, respectively), and MMP-2 in echocardiographically normal patients (p = 0.002). In conclusion, BAV patients with isolated severe aortic stenosis demonstrated a distinct plasma MMP/TIMP pattern, which might be utilized as circulating biomarkers for early detection of aortic dilatation.



Similar content being viewed by others
References
Verma S, Siu SC (2014) Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 370:1920–1929
Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, Malaisrie SC, McCarthy P, Collins J, Carr J, Markl M (2014) Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 129:673–682
Tadros TM, Klein MD, Shapira OM (2009) Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation 119:880–890
Wang Y, Wu B, Dong L, Wang C, Shu X (2013) Type A aortic dissection in patients with bicuspid or tricuspid aortic valves: a retrospective comparative study in 288 Chinese patients. Eur J Cardiothorac Surg 44:172–177
Sciacca S, Pilato M, Mazzoccoli G, Pazienza V, Vinciguerra M (2013) Anti-correlation between longevity gene SirT1 and Notch signaling in ascending aorta biopsies from patients with bicuspid aortic valve disease. Heart Vessel 28:268–275
Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM Jr, Elefteriades JA, Coady MA (2007) Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 83:1338–1344
Nathan DP, Xu C, Plappert T, Desjardins B, Gorman JH 3rd, Bavaria JE, Gorman RC, Chandran KB, Jackson BM (2011) Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg 92:1384–1389
Meierhofer C, Schneider EP, Lyko C, Hutter A, Martinoff S, Markl M, Hager A, Hess J, Stern H, Fratz S (2013) Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Heart J Cardiovasc Imaging 14:797–804
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41:271–290
Kaunas R, Kang H, Bayless KJ (2011) Synergistic regulation of angiogenic sprouting by biochemical factors and wall shear stress. Cell Mol Bioeng 4:547–559
Tzemos N, Lyseggen E, Silversides C, Jamorski M, Tong JH, Harvey P, Floras J, Siu S (2010) Endothelial function, carotid-femoral stiffness, and plasma matrix metalloproteinase-2 in men with bicuspid aortic valve and dilated aorta. J Am Coll Cardiol 55:660–668
Barker AJ, Markl M, Bürk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F (2012) Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 5:457–466
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD (2014) 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:e57–e185
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology; American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine (2010) 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease. J Am Coll Cardiol 55:e27–e129
Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U, Taylor J, Zollikofer C, Klein WW, Mulder B, Providencia LA, Task Force on Aortic Dissection, European Society of Cardiology (2001) Diagnosis and management of aortic dissection. Eur Heart J 22:1642–1681
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M, American Society of Echocardiography, European Association of Echocardiography (2009) Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 22:1–23
Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B (2003) Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med 349:343–349
Guntheroth WG (2008) A critical review of the American College of Cardiology/American Heart Association practice guidelines on bicuspid aortic valve with dilated ascending aorta. Am J Cardiol 102:107–110
van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8:50–60
Bissell MM, Hess AT, Biasiolli L, Glaze SJ, Loudon M, Pitcher A, Davis A, Prendergast B, Markl M, Barker AJ, Neubauer S, Myerson SG (2013) Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 6:499–507
Walther T, Schubert A, Falk V, Binner C, Kanev A, Bleiziffer S, Walther C, Doll N, Autschbach R, Mohr FW (2001) Regression of left ventricular hypertrophy after surgical therapy for aortic stenosis is associated with changes in extracellular matrix gene expression. Circulation 104:I54–I58
Fujiwara T, Matsunaga T, Kameda K, Abe N, Ono H, Higuma T, Yokoyama J, Hanada H, Osanai T, Okumura K (2007) Nicorandil suppresses the increases in plasma level of matrix metalloproteinase activity and attenuates left ventricular remodeling in patients with acute myocardial infarction. Heart Vessel 22:303–309
Arjunon S, Rathan S, Jo H, Yoganathan AP (2013) Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng 41:1331–1346
Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH 3rd, Spinale FG, Gorman RC (2007) Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg 133:1028–1036
Phillippi JA, Green BR, Eskay MA, Kotlarczyk MP, Hill MR, Robertson AM, Watkins SC, Vorp DA, Gleason TG (2014) Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg 147:1056–1064
Gambillara V, Montorzi G, Haziza-Pigeon C, Stergiopulos N, Silacci P (2005) Arterial wall response to ex vivo exposure to oscillatory shear stress. J Vasc Res 42:535–544
Platt MO, Xing Y, Jo H, Yoganathan AP (2006) Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J Heart Valve Dis 15:622–629
Ikonomidis JS, Ivey CR, Wheeler JB, Akerman AW, Rice A, Patel RK, Stroud RE, Shah AA, Hughes CG, Ferrari G, Mukherjee R, Jones JA (2013) Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J Thorac Cardiovasc Surg 145:1326–1333
Sultan S, Gosling M, Nagase H, Powell JT (2004) Shear stress-induced shedding of soluble intercellular adhesion molecule-1 from saphenous vein endothelium. FEBS Lett 564:161–165
Milkiewicz M, Kelland C, Colgan S, Haas TL (2006) Nitric oxide and p38 MAP kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol 208:229–237
Yamane T, Mitsumata M, Yamaguchi N, Nakazawa T, Mochizuki K, Kondo T, Kawasaki T, Murata S, Yoshida Y, Katoh R (2010) Laminar high shear stress up-regulates type IV collagen synthesis and down-regulates MMP-2 secretion in endothelium. A quantitative analysis. Cell Tissue Res 340:471–479
Kilickesmez KO, Abaci O, Kocas C, Yildiz A, Kaya A, Okcun B, Kucukoglu S (2012) Dilatation of the ascending aorta and serum alpha 1-antitrypsin level in patients with bicuspid aortic valve. Heart Vessel 27:391–397
Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ (2014) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. doi:10.1136/annrheumdis-2013-204837
Takata M, Amiya E, Watanabe M, Ozeki A, Watanabe A, Kawarasaki S, Nakao T, Hosoya Y, Uno K, Saito A, Murasawa T, Ono M, Nagai R, Komuro I (2014) Brachial artery diameter has a predictive value in the improvement of flow-mediated dilation after aortic valve replacement for aortic stenosis. Heart Vessel. doi:10.1007/s00380-014-0475-x
Wang Z, Juttermann R, Soloway PD (2000) TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem 275:26411–26415
Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY (2001) MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res 263:209–223
Mizia-Stec K, Lasota B, Mizia M, Chmiel A, Adamczyk T, Chudek J, Gasior Z (2013) Copeptin constitutes a novel biomarker of degenerative aortic stenosis. Heart Vessel 28:613–619
Spinale FG, Stolen CM (2013) Biomarkers and heart disease: what is translational success? J Cardiovasc Transl Res 6:447–448
Naito Y, Tsujino T, Lee-Kawabata M, Matsumoto M, Ezumi A, Nakao S, Goda A, Ohyanagi M, Masuyama T (2009) Matrix metalloproteinase-1 and -2 levels are differently regulated in acute exacerbation of heart failure in patients with and without left ventricular systolic dysfunction. Heart Vessel 24:181–186
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Grant No. 81300232), the Shanghai Young Scientific ‘Phosphor’ Foundation (Grant No. 12QA1400700).
Conflicts of interest
No conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Wang and B. Wu contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Wang, Y., Wu, B., Dong, L. et al. Circulating matrix metalloproteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis. Heart Vessels 31, 189–197 (2016). https://doi.org/10.1007/s00380-014-0593-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0593-5