Abstract
Statins reduce cardiovascular morbidity and mortality from coronary artery disease (CAD). However, the effects of statin therapy in patients with CAD and chronic kidney disease (CKD) remain unclear. Within a single hospital-based cohort in the Shinken Database 2004–2010 comprising all patients (n = 15,227) who visited the Cardiovascular Institute, we followed patients with CKD and CAD after percutaneous coronary intervention (PCI). A major adverse cardiovascular and cerebrovascular event (MACCE) was defined by composite end points, including death, myocardial infarction, cerebral infarction, cerebral hemorrhage, and target lesion revascularization. A total of 391 patients were included in this study (median follow-up time 905 ± 679 days). Of these, 209 patients used statins. Patients with statin therapy were younger than those without. Obesity and dyslipidemia were more common, and the glomerular filtration rate (GFR) was significantly higher, in patients undergoing statin treatment. MACCE and cardiac death tended to be less common, and all-cause death was significantly less common, in patients taking statins. Multivariate analysis showed that low estimated GFR, poor left ventricular ejection fraction, and the absence of statin therapy were independent predictors for all-cause death of CKD patients after PCI. Statin therapy was associated with reduced all-cause mortality in patients with CKD and CAD after PCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) increases the risk of cardiovascular mortality and morbidity [1, 2]. Furthermore, patients with coronary artery disease (CAD) are believed to have more frequently impaired renal function, and reduced renal function may result in worse clinical outcomes after revascularization therapy [2–5]. Statins reduce cardiovascular morbidity and mortality from CAD as a primary [6–8] and secondary [9–13] preventative. In addition, aggressive statin treatment reduces future cardiovascular events, even in CKD patients [14, 15].
CAD is becoming more common in the elderly as the mean age of the population increases in Japan [16]. Moreover, we previously reported that cardiovascular events, including cardiac death, nonfatal myocardial infarction (MI), or readmission for heart failure, increase significantly with declining estimated glomerular filtration rate (eGFR) [17]. In Japanese patients with acute MI, early lipid-lowering treatment with statins decreases recurring cardiovascular events—in particular, congestive heart failure [18]. Intensive statin treatment appears to be more effective than standard statin doses after acute coronary syndrome [19, 20] and stable angina pectoris [21]. However, the standard statin doses used in previous studies conducted in Western countries were larger than the standard doses approved in Japan. Individuals of Asian descent are thought to be more responsive than Caucasians to therapeutic drugs, and the respective standard doses appear to provide the same benefit to Asian patients [22]. We previously reported that statin therapy initiated early after the diagnosis of CAD may decrease the risk of fatal events in Japanese CAD patients [23]; however, the impact of statin therapy in patients with CKD and CAD after percutaneous coronary intervention (PCI) was less well defined.
A prospective cohort study, the Shinken Database, was designed to investigate the morbidity and mortality of Japanese patients with CAD who underwent PCI. In this study, using this database we evaluated the effects of statin treatment in patients with CKD and CAD after PCI.
Patients and methods
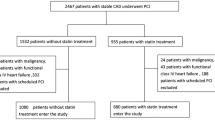
Study patients and protocols
The Shinken Database comprises all new patients who visit the Cardiovascular Institute in Tokyo, Japan (“Shinken” is an abbreviated name in Japanese for the name of the hospital), and excludes patients with active cancer and foreign travelers. The principal aim of this hospital-based database is surveillance of the prevalence and prognosis of cardiovascular diseases in the urban areas of Japan [24]. The registry began in June 2004, and thereafter patients have continually been registered in the database on an annual basis. The data in the present study were derived from this database between June 2004 and March 2011 (Shinken Database 2004–2010), including 15,227 new patients. Those patients with CKD and CAD who underwent PCI (n = 391) were enrolled in this study. PCI procedures including stent selection were performed by experienced operators. The following data were obtained: age, gender, height, body weight, prior history of MI, PCI, and coronary artery bypass graft (CABG), coronary risk factors, laboratory data, types of the implanted stents (bare-metal stent and/or drug-eluting stent), and medications at primary PCI. Ultrasound cardiography was routinely performed at the time of PCI.
Patient follow-up
The health status, incidence of cardiovascular events, and mortality are maintained in the database through linking with the medical records of the hospital, and prognostic study documents are sent annually to those who discontinued hospital visits or were referred to other hospitals.
In the present data analysis, data from after April 1, 2011 were excluded. The end of the follow-up period was therefore defined by: (1) the date of death, if the date was prior to March 31, 2011; (2) the final hospital visit or the final response to our prognostic study documents prior to March 31, 2011; or (3) March 31, 2011, when the date of death, the final hospital visit, or the final response to our study documents was later than April 1, 2011.
Ethics
The ethical committee of the Cardiovascular Institute granted ethical permission for this study, and all patients provided written informed consent.
Definitions
We confirmed the deaths of study patients in the medical records of our hospital or by the information obtained from follow-up. Body mass index (BMI) was calculated at initial PCI by dividing the patient’s measured weight (in kilograms) by the square of the height (in meters); obesity was defined as a BMI of ≥25 kg/m2. GFR was calculated using the GFR equation designed for the Japanese population: GFR = 194 × (serum creatinine)−1.094 × (age)−0.287 × (0.739, if female) [25]. CKD was defined as eGFR <60 ml/min/1.73 m2. Target lesion revascularization (TLR) is defined as any repeat revascularization procedure (percutaneous or surgical) of the original target lesion site, including the stented plus edge segments (typically 5 mm proximal and distal to the stent). A major adverse cardiovascular and cerebrovascular event (MACCE) was defined as a composite end point including all-cause death, MI, cerebral infarction, cerebral hemorrhage, and TLR.
Statistical analysis
Categorical and consecutive data are presented as number (%) and mean ± standard deviation (SD), respectively. The unpaired t test was used for comparison of consecutive variables between the two groups. Chi-square analysis was used to compare categorical variables. Long-term event-free survival was estimated using Kaplan–Meier curves, and the log-rank test was used to assess the significance of differences between patients with and without statin treatment. Univariate Cox regression analysis was used to identify cofactors with significant effects on all-cause death in CKD and CAD patients after PCI. Multivariate Cox regression analysis was performed to determine the independent prognostic factors for all-cause death of CKD and CAD patients after PCI. A probability value of less than 0.05 was considered to indicate a statistically significant difference. These analyses were performed using SPSS software (SPSS, Chicago, IL, USA), version 19.0.
Results
Patients’ characteristics
Of 391 patients, 209 (54 %) were taking statins. The median follow-up period was 905 ± 679 days. Patients taking statins were younger than patients without statins (68.7 ± 10.1 vs 72.0 ± 9.9 years, P = 0.001). Obesity (43.3 % vs 28.2 %, P = 0.001) and dyslipidemia (73.7 % vs 34.6 %, P < 0.001) were more common in patients taking statins than in those who were not. Patients taking statins had significantly higher eGFR (47.3 ± 12.6 vs 42.0 ± 17.7 ml/min/1.73 m2, P = 0.001). Triglyceride levels were significantly higher in the patients taking statins (151.7 ± 111.0 vs 127.8 ± 79.1 mg/dl, P = 0.015). Patients taking statins more commonly used dual antiplatelet therapy (98.6 % vs 91.8 %, P = 0.001; Table 1).
Echocardiographic findings
Ultrasound cardiography showed that the left ventricular ejection fraction (LVEF) was comparable between patients taking and not taking statins (59.4 % ± 14.5 % vs 58.7 % ± 16.3 %, P = 0.688; Table 1).
Angiographic findings
Coronary angiography showed that the prevalence of left main trunk (LMT) disease (11.0 % vs 8.2 %, P = 0.358) and multivessel disease (MVD) (68.9 % vs 66.5 %, P = 0.610) were comparable between patients using and not using statins (Table 1).
Clinical outcomes
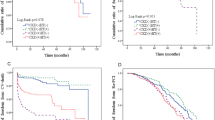
All-cause death and cardiac death occurred in 9 and 3 patients taking statins, respectively, and 29 and 10 patients who were not taking statins (Table 2). Kaplan–Meier curves and the log-rank test revealed that the frequencies of MACCE (P = 0.188) and cardiac death (P = 0.052) tended to be lower in patients using statin therapy than in those who did not, and the frequency of all-cause death was significantly lower in patients taking statins than in those who did not (P = 0.001). The frequencies of MI (P = 0.662), and TLR (P = 0.507) were comparable between patients using and not using statins (Fig. 1).
Kaplan–Meier curves for the major adverse cardiovascular and cerebrovascular event (MACCE)-free survival rate (a), all-cause death-free survival rate (b), cardiac death-free survival rate (c), myocardial infarction (MI)-free survival rate (d), and target lesion revascularization (TLR)-free survival rate (e)
Predictors of death
Univariate Cox regression analysis showed that obesity, acute coronary syndrome (ACS), eGFR, glucose levels, LVEF, statins, diuretics, and aldosterone antagonists were associated with death (from all causes) in CKD patients after PCI (Table 3). Multivariate Cox regression analysis, including the significant predictors above and the marginally significant ones (P < 0.10) (including age, hypertension, and dyslipidemia) in the univariate model, showed that low eGFR, poor LVEF, and the absence of statin treatment were independent predictors of death in CKD patients after PCI (Table 4).
Discussion
The present study showed that statin treatment reduced all-cause mortality of Japanese CAD patients after PCI complicated with CKD. Multivariate Cox regression analysis showed that the absence of statin treatment, as well as low eGFR and poor LVEF, was an independent predictor of all-cause death of CKD patients after PCI.
Statins are promising agents for CAD, as supported by a plethora of clinical evidence [7, 9, 11]. In Western countries, statins are administered to 80 %–90 % of CAD patients [26]. By contrast, in the present study CKD patients received statins after PCI in 54 % of cases. Administration of statin therapy was determined by the attending physician, based on the guidelines. Although statins were prescribed for patients, they were often not taken or discontinued for different reasons. These findings should reassure physicians that the use of statin treatment is critical for patients with CAD after PCI to prevent adverse outcomes. Because underuse of statins in patients with CKD may relate to potential toxicity concerns in patients with impaired renal function, the recommendation of the National Lipid Association Statins Safety Assessment Task Force [27] indicated that CKD should not preclude the use of statins.
It was controversial that treatment with statin might prevent future cardiac events of patients with renal dysfunction [28–31]. In the 4D study, atorvastatin had no statistically significant effect on the composite primary end point of cardiovascular death, nonfatal MI, and stroke in patients with diabetes receiving hemodialysis, suggesting that the benefit of statin treatment was limited when intervention with statins was postponed until patients had reached end-stage renal disease [32]. In this study, the number of patients receiving hemodialysis was only 8.7 % (34/391) and most of the study patients had mild CKD. Hence, early statin administration in CKD patients might provide clinical benefit. These findings should reassure physicians that the use of statin treatment is critical for patients with CAD after PCI in preventing adverse outcomes. There may thus be room for further improvement of clinical outcomes by increased use of statins.
To our knowledge, limited data have shown the effects of statin therapy for unselected Japanese CKD patients with CAD who underwent PCI with a broad range of lipid levels; the present study therefore newly demonstrates importance of statin treatment by revealing a significant reduction in all-cause mortality of CKD patients. However, we do not fully understand why the statin treatment reduced the all-cause mortality rate in patients with CKD and CAD who underwent PCI. Statins are known to have pleiotropic effects, including improving endothelial function [33], decreasing platelet activity [33], and attenuating inflammation [34, 35], which may contribute to the decreased mortality rate in CAD and CKD patients.
We found a discrepancy between all-cause mortality and adverse cardiac events in the present study. Previous studies reported that TLR and other cardiovascular events are reduced by statin treatment. This was not observed in the present study. The effect of statin treatment on plaque regression acts in a dose-dependent manner, which may underlie this discrepancy between the present study and previous ones conducted in Western countries. In addition, the lipid profiles of subjects in this study were almost within normal range. Because baseline levels of low-density lipoprotein cholesterol may alter the overall benefit of statins, it is possible that statins, used in doses approved for Japanese subjects, were not sufficient to reduce the restenosis rate or atherosclerotic burden during the follow-up period. Furthermore, PCI was performed in our institute using a careful stent-deployment technique with routine use of intravascular ultrasound. Although the pleiotropic effects of statins can contribute to reduced mortality, the reasons for the discrepancy between mortality and morbidity in our study are not fully explained. Further studies are required to investigate the mechanisms by which statin treatment may prevent fatal events.
Study limitations
We recognize that the present study had several limitations. First, this study reflects the experience of a single center, and the number of patients in the study was small. The statistical power may not be sufficient for negative data to be conclusive. Accordingly, more studies in a larger cohort are needed. Second, we divided patients into two groups, a statin group and a nonstatin group, irrespective of statin types and doses. Further study will be needed to clarify the appropriate types and doses of statin treatment for CAD and CKD patients. Finally, cholesterol profiles and medications were not assessed during follow-up, and some patients in the nonstatin group may have subsequently begun to take statins, while others in the statin group may have discontinued the treatment. However, this misclassification bias would have led us to underestimate the true effects of statin treatment. Despite these limitations, we believe that our findings provide valuable information to inform future treatment for patients with CKD and CAD after PCI, because the target population is representative of patients seen in daily clinical practice and the statin doses in this study were consistent with those used in clinical settings in Japan.
Conclusion
Statin treatment reduced all-cause mortality in Japanese CKD and CAD patients who underwent PCI. The absence of statin treatment, as well as poor left ventricular function and reduced renal function, was an independent predictor for all-cause death of CKD patients, suggesting a crucial role of statin treatment in long-term clinical outcomes of CKD and CAD patients.
References
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G (2003) National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–147
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108:2154–2169
Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M (2006) Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ 332:1426
Wison S, Foo K, Cunningham J, Cooper J, Deaner A, Knight C, Ranjadayalan K, Timmis AD (2003) Renal function and risk stratification in acute coronary syndromes. Am J Cardiol 91:1051–1054
Reinecke H, Trey T, Matzkies F, Fobker M, Breithardt G, Schaefer RM (2003) Grade of chronic renal failure, and acute and long-term outcome after percutaneous coronary interventions. Kidney Int 63:696–701
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333:1301–1307
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359:2195–2207
Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D (2008) Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 52:1769–1781
(1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383–1389
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E (1996) The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335:1001–1009
(1998) Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339:1349–1357
Stenestrand U, Wallentin L (2001) Early statin treatment following acute myocardial infarction and 1-year survival. JAMA 285:430–436
Aronow HD, Topol EJ, Roe MT, Houghtaling PL, Wolski KE, Lincoff AM, Harrington RA, Califf RM, Ohman EM, Kleiman NS, Keltai M, Wilcox RG, Vahanian A, Armstrong PW, Lauer MS (2001) Effect of lipid-lowering therapy on early mortality after acute coronary syndromes: an observational study. Lancet 357:1063–1068
Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK (2008) Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: the TNT (Treating to New Targets) study. J Am Coll Cardiol 51:1448–1454
Ridker PM, MacFadyen J, Cressman M, Glynn RJ (2010) Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention—an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 55:1266–1273
Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Arima H, Tanaka K, Nakamura H, Okubo K, Iida M (2003) Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke 34:2349–2354
Nakamura M, Yamashita T, Yajima J, Oikawa Y, Ogasawara K, Kirigaya H, Sagara K, Koike A, Sawada H, Aizawa T (2009) Impact of reduced renal function on prognosis in Japanese patients with coronary artery disease: a prospective cohort of Shinken Database 2007. Hypertens Res 32:920–926
Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, Sakaino N, Kitagawa A (2006) Effects of early statin treatment on symptomatic heart failure and ischemic events after acute myocardial infarction in Japanese. Am J Cardiol 97:1165–1171
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350:1495–1504
Murphy SA, Cannon CP, Wiviott SD, de Lemos JA, Blazing MA, McCabe CH, Califf RM, Braunwald E (2007) Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the Aggrastat to Zocor and Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 trials). Am J Cardiol 100:1047–1051
LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK (2005) Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352:1425–1435
Liao JK (2007) Safety and efficacy of statins in Asians. Am J Cardiol 99:410–414
Nakamura M, Yamashita T, Yajima J, Oikawa Y, Ogasawara K, Sagara K, Koike A, Kirigaya H, Nagashima K, Otsuka T, Uejima T, Funada R, Matsuno S, Suzuki S, Sawada H, Aizawa T (2011) Impact of early statin initiation on secondary prevention in Japanese patients with coronary artery disease. J Cardiol 57:172–180
Suzuki S, Yamashita T, Ohtsuka T, Sagara K, Uejima T, Oikawa Y, Yajima J, Koike A, Nagashima K, Kirigaya H, Ogasawara K, Sawada H, Aizawa T (2008) Prevalence and prognosis of patients with atrial fibrillation in Japan: a prospective cohort of Shinken Database 2004. Circ J 72:914–920
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA (2011) Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA 305:1882–1889
Kasiske BL, Wanner C, O’Neill WC (2006) An assessment of statin safety by nephrologists. Am J Cardiol 97:82C–85C
Seliger SL, Weiss NS, Gillen DL, Kestenbaum B, Ball A, Sherrard DJ, Stehman-Breen CO (2002) HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int 61:297–304
Cosio FG, Pesavento TE, Pelletier RP, Henry M, Ferguson RM, Kim S, Lemeshow S (2002) Patient survival after renal transplantation III: the effects of statins. Am J Kidney Dis 40:638–643
Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, Gronhagen-Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambuhl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR (2003) Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 361:2024–2031
Tonelli M, Isles C, Curhan GC, Tonkin A, Pfeffer MA, Shepherd J, Sacks FM, Furberg C, Cobbe SM, Simes J, Craven T, West M (2004) Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 110:1557–1563
Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E (2005) Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353:238–248
Dupuis J, Tardif JC, Cernacek P, Theroux P (1999) Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation 99:3227–3233
Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E (1999) Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 100:230–235
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ (2009) Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 373:1175–1182
Acknowledgments
We thank Shiro Ueda and Nobuko Ueda of Medical Edge Co., Ltd., for assembling the Clinical Study Supporting System (CliSSS) database. We also thank Ineko Hayakawa, Hiroaki Arai, and Hiroshi Aoki for data management and system administration, and all staff in the intervention laboratory in our institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kaneko, H., Yajima, J., Oikawa, Y. et al. Effects of statin treatment in patients with coronary artery disease and chronic kidney disease. Heart Vessels 29, 21–28 (2014). https://doi.org/10.1007/s00380-013-0325-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-013-0325-2