Abstract
The purpose was to study optimum timing of continuous veno-venous hemodialysis (CVVHD) for acute renal failure (ARF) after cardiac surgery. CVVHD was performed in two groups [elapsed time between urine output (UO) <0.5 ml/kg/h and dialysis of no more than 12 h in group A and >12 h in group B] with a total of 58 adult patients. Survivors in groups A and B were entered into groups A1 and Bl, respectively. Compared to group A, the acute physiology and chronic health evaluation III score, peak values of urea and creatinine before CVVHD, major complications, period of ICU and hospitalization were significantly higher in group B. In-hospital mortality in group B was significantly higher than that in group A (37.5 vs. 8.8%, p = 0.02). Kaplan-Meier curves confirmed significantly better postoperative survival in group A (χ 2 = 6.966, p = 0.008). Time elapse from UO < 0.5 ml/kg/h until dialysis among the survivors was significantly lower than that among the dead (12.0 ± 6.2 vs. 20.8 ± 9.1 h, p = 0.0002). Additionally, duration of dialysis, length of ICU stay, duration of ventilator support and time elapse from dialysis until UO > 1 ml/kg/h were significantly higher in group B1 as compared to those in group A1. All of them correlated positively with the time elapse from UO < 0.5 ml/kg/h until dialysis. Early beginning of CVVHD is extremely important.
Similar content being viewed by others
Introduction
Acute renal failure (ARF) is a well-known complication of cardiac surgery. Though severe ARF (requiring renal replacement therapy) occurs in 2–5% of patients [1, 2], the mortality rate in those patients a decade ago was reported to be as high as 90% [3]. More recently, continuous venous-venous hemodialysis (CVVHD) has been introduced, which could circumvent the hemodynamic instability associated with intermittent hemodialysis and its limited ability to control the state of patients’ volume. Despite these developments and modified renal replacement therapy modalities, severe ARF following cardiac surgery still had a high mortality [4, 5]. One important factor contributing towards the latter was that hemodialysis was not performed on time in cardiac surgery patients who developed ARF postoperatively. The promise indicated by successful interventions in experimental models suggested that the proposed intervention should come early, possibly within 24–48 h after induction of renal injury [6, 7]. However, it is extremely difficult to translate early interventions into clinical trials as it is difficult to anticipate renal dysfunction and also the use of surrogate markers of glomerular filtration rate as current indicators of acute renal dysfunction leads to a significant delay in the diagnosis of ARF. Consequently, optimum timing of hemodialysis in the treatment of ARF following cardiac surgery has been inconclusive. This study reviewed 58 patients undergoing CVVHD following cardiac surgery in our medical center from April 2004 to March 2009 to evaluate optimum timing of CVVHD in the treatment of ARF following cardiac surgery.
Materials and methods
Selection criteria
The original selection criteria included normal preoperative blood urea nitrogen (BUN) and serum creatinine, no history of chronic renal failure, oliguria or anuria before CVVHD that did not respond to fluid replacement or hemodynamic resuscitation or furosemide administration, and duration of CVVHD of more than 12 h. Patients who were readmitted after discharge, those who were discharged against advice, and those who died within 24 h of surgery were excluded.
Postoperative ARF was described as postoperative urinary output of less than 0.5 ml/kg/h, and a 50% increase in baseline BUN and serum creatinine levels.
Based on our hospital’s protocol for initiation of dialysis in ARF, we opted for 12 h as the cutoff point in this study.
Research methods
All selected patients were divided either into group A or B according to the time elapsed from oliguria (urine output < 0.5 ml/kg/h) until initiation of CVVHD. In group A the time elapse was no more than 12 h, whereas in group B it was more than 12 h. Finally, the survivors in groups A and B were entered into groups A1 and B1, respectively. Data were obtained from the patients’ files as well as from our cardiac surgery database. Preoperative parameters of the patients included age, gender, recent smoking (within 4 weeks of surgery), diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), recent myocardial infarction (MI) (evidence of MI in the last 30 days before surgery), congestive heart failure (NYHA class III and IV), left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and baseline urea and creatinine levels. Surgical variables included redo surgery, emergency surgery, surgery type, duration of cardiopulmonary bypass (CPB), aortic cross-clamp (ACC) time, requirement of deep hypothermic circulatory arrest and low mean perfusion pressure (mean pressure <50 mmHg for more than 30 min). Postoperative variables included postoperative hypotension (systolic blood pressure less than 90 mmHg for more than 1 h), blood or blood product transfusion, fresh frozen plasma transfusion, peak values of urea and creatinine levels before CVVHD and potassium values immediately before CVVHD. Major postoperative complications included re-exploration for bleeding, postoperative myocardial infarction (new Q-wave infarction within 48 h after surgery), stroke (new permanent neurological event; early stroke: within 24 h; delayed stroke >24 h postoperatively), gastrointestinal bleeding, pneumonia, ventilator support >72 h, arrhythmia, low cardiac output, sepsis and multiple organ failure. Peak values of acute physiology and chronic health evaluation III score (APACHE III score) before dialysis were recorded in all selected patients. CVVHD, duration of ventilator support, length of intensive care unit (ICU) stay and time elapse from the initiation of CVVHD until urine output of >1 ml/kg/h were recorded in the patients who survived.
Renal support
The following criteria were employed for the initiation of CVVHD: anuria, high levels of serum potassium despite diuretic and inotropic support, development of hypervolemia, and acidosis. All patients received dopamine, beginning as from the immediate postoperative period and continuing up to the first postoperative day at the renal dose of 3 mg/kg/min. Dopamine dose was increased (4–10 mg/kg/min) in patients who had low blood pressure due to pump insufficiency. Calculations of volume infusion in the intraoperative and postoperative periods were performed with the help of pulmonary capillary wedge and central venous pressures.
Vascular access for CVVHD was obtained using a temporary central venous double lumen catheter inserted into the femoral vein and connected to the extra-corporeal circuit. Blood flow rate was maintained at 100 ml/min. Flow rates of the dialysate and diafiltrate were preset at 900 and 1,000 ml/h, respectively. Flow rate of removal of plasma fluid was approximately 100 ml/h according to the above preset values. Heparin, as an anticoagulant, was administered at 10–12 UI/kg/h through the predialyzer compartment of the circuit. The activated coagulation time (ACT), which was monitored at least every 8 h, was maintained between 140 and 160 s. The patients started to be weaned off from CVVHD when urine output of more than 1 ml/kg/h was obtained and when a gradual decrease in BUN and serum creatinine levels were noted.
Statistics
Statistical analysis was performed using the SPSS13.0 statistical software package. Continuous variables are presented as mean ± standard deviation. Comparison between groups A and B was achieved using the unpaired t test or t′ test according to the homogeneity test for variance. Chi-square analysis or Fisher’s exact test was used to compare quantitative data. Postoperative survival analysis was performed by the Kaplan-Meier method with log-rank test for group comparisons. Correlation between the time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD and duration of CVVHD, duration of ventilator support, length of ICU stay as well as the time elapse from the initiation of CVVHD to urine output >1 ml/kg/h was analyzed using the Spearman’s rank correlation analysis. Statistical significance was accepted at a level of p < 0.05.
Results
Study population
Fifty-eight adult patients who underwent CVVHD after cardiac surgery, from a single surgeon’s practice, spanning over a 5-year period from April 2004 to March 2009, were entered into this study. Four patients who underwent emergent spiral CT angiography and ten patients who underwent emergent coronary artery angiography were administered radiographic contrast agents during the peri-operation period. Postoperative hypotension and long duration of cardiopulmonary bypass could have been the two main causes of postoperative ARF despite the use of contrast agent in some of the patients who initially had normal preoperative BUN and serum creatinine, as well as no history of chronic renal failure.
All selected patients underwent between 26 and 160 consecutive hours of CVVHD; no patient required permanent dialysis; 46 patients survived with a mortality rate of 20.7%. The causes of death were as follows: sepsis (n = 7), low cardiac output (n = 3), arrhythmia (n = 1) and gastrointestinal bleeding (n = 1). Time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD was significantly lower among the survivors (12.0 ± 6.2 h among the survivors vs. 20.8 ± 9.1 h among the dead, t = 3.95, p = 0.0002).
Comparison of parameters between the two groups
Clinical data of groups A and B are shown in Table 1. No significant differences were observed between groups A and B as far as preoperative parameters, surgical variables, postoperative variables, baseline BUN and serum creatinine are concerned. However, peak values of BUN and serum creatinine before CVVHD as well as potassium values immediately before CVVHD were significantly higher in group B. Both the time elapse from surgery until the initiation of CVVHD and the time elapse from oliguria (urine output < 0.5 ml/kg/h) until the initiation of CVVHD were significantly higher in group B. Also, the pre-dialysis APACHE III score was significantly higher in group B.
Intra- and postoperative intravenous use of antibiotics, analgesic, inotropic agents and furosemide are shown in Table 2. Doses of dopamine, dobutamine and adrenaline in group B were significantly higher than those in group A. The total amount of furosemide administered between surgery and dialysis was significantly higher in group B.
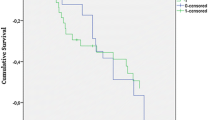
As shown in Fig. 1, Kaplan-Meier curves confirmed a significantly better postoperative survival in group A (χ 2 = 6.966, p = 0.008).
Postoperative complications
Major postoperative complications and the number of in-hospital deaths are shown in Table 3. Re-exploration for bleeding, postoperative myocardial infarction, gastrointestinal bleeding, stroke and arrhythmia were similar between the two groups. However, other major complications such as postoperative pneumonia, prolonged ventilation time (>72 h), postoperative low cardiac output, sepsis, multiple organ failure, ICU and hospitalization stay as well as in-hospital mortality were significantly higher in group B as compared to group A.
Comparison of parameters between survivors in the two groups
Duration of CVVHD, length of ICU stay, duration of ventilator support and time elapse from the initiation of CVVHD until urine output >1 ml/kg/h in group B1 were significantly higher than those in group A1, as shown in Table 4. In addition, the Spearman’s rank correlation analysis indicated that the time elapse from urine output < 0.5 ml/kg/h until the initiation of CVVHD correlated positively with duration of CVVHD, time elapse from the initiation of CVVHD until urine output > 1 ml/kg/h, length of ICU stay and duration of ventilator support: r 1 = 0.773 (p < 0.05), r 2 = 0.635 (p < 0.05), r 3 = 0.572 (p < 0.05) and r 4 = 0.357 (p < 0.05), respectively.
Discussion
Acute renal failure is one of the major complications after cardiopulmonary bypass for open heart operations [1, 2]. Preoperative renal insufficiency and postoperative hypotension are the most important independent risk factors for ARF in postcardiac surgical patients [8, 9]. Mild-to-moderate ARF following cardiac surgery may be reversible through medication. However, severe ARF, which usually combines with multiple organ dysfunction syndrome, low cardiac output, endotoxemia, hypervolemia, hyperkalemia and acidosis, might be irreversible if renal replacement therapy is not performed appropriately and on time [5, 10, 11]. CVVHD, which can circumvent the hemodynamic instability associated with intermittent hemodialysis and its limited ability to control the state of the patients’ volume, is playing an increasingly important role in the treatment of ARF following cardiac surgery [5, 12–14]. However, optimum timing of CVVHD in the treatment of ARF after cardiac surgery has been inconclusive.
A study [15] in 2004 showed that early renal replacement therapy during the first postoperative day, this time with continuous veno-venous hemofiltration, achieved a significantly lower mortality of 22% when compared with the same treatment started 2½ days after surgery when the mortality was 43%. Demirkilic [14] reported performing continuous veno-venous hemodiafiltration when urine output was less than 100 ml within 8 consecutive hours with no response to 50 mg furosemide, and the overall hospital mortality rate was merely 23.5%. Although these different studies can not be compared because they used different criteria for defining acute renal injury and instituting renal replacement therapy, renal replacement therapy was recommended to be performed as early as possible for cardiac surgery patients who developed postoperative ARF [16]. In this study, the time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD and the time elapse from surgery until the initiation of CVVHD in group B were double compared to those in group A. Peak values of BUN and serum creatinine before CVVHD were significantly higher in group B, meaning patients in group B had more serious impairment of renal function before CVVHD compared to those in group A. The doses of dopamine, dobutamine and adrenaline before CVVHD were significantly higher in group B, meaning patients in group B had worse hemodynamics before CVVHD as compared to those in group A. The APACHE III scores before CVVHD were significantly higher in group B, meaning patients in group B had worse conditions before CVVHD compared to those in group A. Major complications such as postoperative pneumonia, prolonged ventilator support, postoperative low cardiac output, sepsis and multiple organ failure and in-hospital mortality were significantly higher in group B. Furthermore, Kaplan-Meier curves displayed a significantly better survival in group A versus group B. So, along with the increase in the time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD, the patients’ conditions increasingly worsened. Also, the major morbidities and in-hospital mortality showed a parallel increase. In addition, as compared to the dead, the time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD was significantly lower among the survivors in whom dialysis was started quite early after surgery. Therefore, the sooner the CVVHD was performed, the higher was the likelihood of reduction in major morbidities and in-hospital mortality. This study confirmed the importance of early dialysis for ARF after cardiac surgery.
This study also indicated that duration of CVVHD, duration of ventilator support, duration of ICU stay and the time elapse from the initiation of CVVHD until urine output >1 m1/kg/h were significantly higher in group B1. The time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD correlated positively with the duration of CVVHD, the time elapsed from the initiation of CVVHD to urine output >1 ml/kg/h, length of ICU stay and duration of ventilator support. Since patients in group A1 had shorter ventilation time, duration of CVVHD and ICU stay, their cost to the hospital was equally and significantly less as compared to those in group B1. So, early dialysis decreased the cost of management. From these points of view, this study further demonstrated the importance of early dialysis for ARF after cardiac surgery.
In this study, 12 patients died, with a mortality rate of 20.7%. Sepsis and low cardiac output were the two major causes of death. The time elapse from urine output <0.5 ml/kg/h until the initiation of CVVHD among the survivors was 12.0 ± 6.2 h, which was significantly lower than that among the dead with the elapsed time of 20.8 ± 9.1 h. Also the number of in-hospital deaths in group B (the time elapse from urine output <0.5 ml/kg/h to the initiation of CVVHD more than 12 h) was significantly higher than that in group A (the time elapse from urine output <0.5 ml/kg/h to the initiation of CVVHD no more than 12 h). Kaplan-Meier curves displayed a significantly better survival in group A versus group B. So, delayed CVVHD contributed to a higher mortality. Use of surrogate markers (BUN and serum creatinine) of the glomerular filtration rate as current indicators of acute renal dysfunction following cardiac surgery leads to a significant delay in the diagnosis of ARF, contributing to delayed renal replacement therapy. In addition, our poor understanding of this complex clinical problem (i.e., ARF after cardiac surgery) may have further contributed to delayed renal replacement therapy. Initially, CVVHD was reserved as a last resort when it became impossible to correct hyperkalemia, leading to a higher mortality. In this study, when CVVHD was performed beyond 12 h after urine output was <0.5 ml/kg/h, the mortality hit 37.5%. It later became apparent that our poor results may have resulted from the late institution of dialysis, so early dialysis was performed for cardiac surgery patients who developed ARF postoperatively. Early renal replacement therapy (time elapse from urine output <0.5 ml/kg/h to the initiation of CVVHD no more than 12 h) achieved a significantly lower mortality of 8.8%.
In this study, no patient required permanent dialysis. All selected patients had normal baseline BUN and serum creatinine levels, without a history of chronic renal failure. ARF following cardiac surgery caused by various factors, especially hypotension, may be temporary and reversible if the appropriate treatment is instituted on time. However, 12 patients died, and we could not confirm whether they would have needed permanent dialysis if they had survived.
The retrospective nature and small sample size were the main limitations of this study. Further studies are recommended to address each of the limitations in this study individually.
References
Llopart T, Lombardi R, Forselledo M, Andrade R (1997) Acute renal failure in open heart surgery. Ren Fail 19:319–323
Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, Paganini EP (2003) ARF after open-heart surgery: influence of gender and race. Am J Kidney Dis 41:742–751
Endre ZH (1995) Post cardiac surgery acute renal failure in the 1990s. Aust N Z J Med 25:278–279
Ostermann ME, Taube D, Morgan CJ, Evans TW (2000) Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med 26:565–571
Bent P, Tan HK, Bellomo R, Buckmaster J, Doolan L, Hart G, Silvester W, Gutteridge G, Matalanis G, Raman J, Rosalion A, Buxton BF (2001) Early and intensive continuous hemofiltration for severe renal failure after cardiac surgery. Ann Thorac Surg 71:832–837
Star RA (1998) Treatment of acute renal failure. Kidney Int 54:1817–1831
Bonventre JV, Weinberg JM (2003) Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14:2199–2210
Suen WS, Mok CK, Chiu SW, Cheung KL, Lee WT, Cheung D, Das SR, He GW (1998) Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology 49:789–800
Liu H, Yu J, Chen F, Li J, Hu D (2007) Inpatients with coronary heart disease have a high prevalence of chronic kidney disease based on estimated glomerular filtration rate (eGFR) in China. Heart Vessels 22:223–228
Behrend T, Miller SB (1999) Acute renal failure in the cardiac care unit: etiologies, outcomes, and prognostic factors. Kidney Int 56:238–243
Ueno H, Nakayama M, Kojima S, Kusuhara K, Nagayoshi Y, Yamamuro M, Nishijima T, Usuku H, Kaikita K, Sumida H, Yamabe H, Sugiyama S, Yoshimura M, Ogawa H (2008) The synergistic combined effect of anemia with high plasma levels of B-type natriuretic peptide significantly predicts an enhanced risk for major adverse cardiac events. Heart Vessels 23:243–248
Lugones F, Chiotti G, Carrier M, Parent D, Thibodeau J, Ducharme B, Cardinal J, Leblanc M (2004) Continuous renal replacement therapy after cardiac surgery. Review of 85 cases. Blood Purif 22:249–255
Bellomo R, Ronco C (1999) Continuous renal replacement therapy in the intensive care unit. Intensive Care Med 25:781–789
Demirkiliç U, Kuralay E, Yenicesu M, Cağlar K, Oz BS, Cingöz F, Günay C, Yildirim V, Ceylan S, Arslan M, Vural A, Tatar H (2004) Timing of replacement therapy for acute renal failure after cardiac surgery. J Card Surg 19:17–20
Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ (2004) Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur J Cardiothorac Surg 26:1027–1031
Manché A, Casha A, Rychter J, Farrugia E, Debono M (2008) Early dialysis in acute kidney injury after cardiac surgery. Interact Cardiovasc Thorac Surg 7:829–832
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, Q., Mei, Y., Wang, X. et al. Timing of continuous veno-venous hemodialysis in the treatment of acute renal failure following cardiac surgery. Heart Vessels 26, 183–189 (2011). https://doi.org/10.1007/s00380-010-0045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-010-0045-9