Abstract
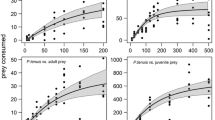
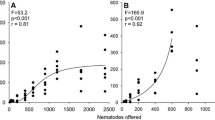
In a series of laboratory experiments, we presented carnivorous Macrobiotus richtersi (Tardigrada, Macrobiotidae) with nematode prey to assess their importance as predator. We investigated consumption rate for (a) different prey densities (10–400 prey individuals), (b) different prey biomasses (22–80 ng), (c) different prey species (Pelodera teres, Rhabditidae, versus Acrobeloides nanus, Cephalobidae) and (d) different environments (2-D agar surface versus 3-D sand fractions of three different textures). M. richtersi consumed up to 4.6 μg nematode prey in 4 h, that is, 43% of the tardigrade’s body mass. Predation rate was positively correlated with prey density. The optimal prey in the present investigation was the biggest prey because it yielded the highest biomass uptake per time. In addition, the size of M. richtersi played an important role in consumption rate. Bacterivorous nematodes reacted differently to attack. Even in a water film on stiff agar where nematode agility was limited, a vigorous undulation reaction of P. teres led to a measurable reduction in consumption rate. A. nanus, in contrast, showed little response to attack. Microcosm experiments with sands of different particle size demonstrated that M. richtersi is able to chase and consume small bacterivorous nematodes in a 3-D soil matrix. However, consumption rate in sand microcosms was significantly reduced compared with pure agar. The sand matrix improved nematode agility and possibly provided small pores as refuge for the nematodes. The lowest consumption rate was observed in fine sand. Effects of predatory tardigrades on nematode numbers in the field are discussed.



Similar content being viewed by others
References
Anderson JM (1995) Soil organisms as engineers: microsite modulation of macroscale processes. In: Jones CG, Lawton JK (eds) Linking species and ecosystems. Chapman and Hall, New York, pp 94–106
Andrássy I (1956) Die Rauminhalts- und Gewichtsbestimmung der Fadenwürmer (Nematoden). Acta Zool 2:1–15
Beier S, Bolley M, Traunspurger W (2004) Predator–prey interactions between Dugesia gonocephala and free-living nematodes. Freshw Biol 49:77–86
Bengtsson J, Wei Zheng D, Agren GI, Persson T (1995) Food webs in soil: an interface between population and ecosystem ecology. In: Jones CG, Lawton JK (eds) Linking species and ecosystems. Chapman and Hall, New York, pp 159–165
Bilgrami AL (1992) Resistance and susceptibility of prey nematodes to predation and strike rate of the predators, Mononchus aquaticus, Dorylaimus stagnalis and Aquatides thornei. Fundam Appl Nematol 15:265–270
Bilgrami AL (1993) Analysis of the predation by Aporcelaimellus nivalis on prey nematodes from different prey trophic categories. Nematologica 39:356–365
Bouwman LA, Hoenderboom GHJ, Van der Maas KJ, De Ruiter PC (1996) Effects of nematophagous fungi on numbers and death rates of bacterivorous nematodes in arable soil. J Nematol 28:26–35
Cogni R, Freitas AVL, Amaral Filho BF (2002) Influence of prey size on predation success by Zelus longipes L. (Het., Reduviidae). J Appl Entomol 126:74–78
Coleman DC, Anderson RV, Cole CV, McClellan JF, Woods LE, Trofymow JA, Elliott ET (1984) Roles of protozoa and nematodes in nutrient cycling. In: Giddens SE, Todd RL (eds) Microbial–plant interactions. ASA, Madison, pp 17–28
De Ruiter PC, Van Veen JA, Moore C, Brussaard L, Hunt HW (1993) Calculation of nitrogen mineralization in soil food webs. Plant Soil 157:263–273
Diehl S, Feißel M (2000) Effects of enrichment on three-level food chains with omnivory. Am Nat 155:200–218
Doncaster CC, Hooper DJ (1961) Nematodes attacked by protozoa and tardigrades. Nematologica 6:333–335
Eibye-Jacobsen J (2001) A new method for making SEM preparations of the tardigrade buccopharyngeal apparatus. Zool Anz 240:309–319
Elliott ET, Anderson RV, Coleman DC, Cole CV (1980) Habitable pore space and microbial trophic interactions. Oikos 35:327–335
Freckman DW (1988) Bacterivorous nematodes and organic-matter decomposition. Agric Ecosyst Environ 24:195–217
Gange AC, Brown VK (2002) Soil food web components affect plant community structure during early succession. Ecol Res 17:217–227
González-Olivares E, Ramos-Jiliberto R (2003) Dynamic consequences of prey refuges in a simple model system: more prey, fewer predators and enhanced stability. Ecol Model 166:135–146
Grootaert P, Jaques A, Small RW (1977) Prey selection in Butlerius sp. (Rhabditida, Diplogasteridae). Meded Fac Landbouwwet Rijksuniv Gent 24:1559–1563
Hallas TE, Yeates GW (1972) Tardigrada of the soil and litter of a Danish beach forest. Pedobiologia 12:287–304
Hohberg K (2003) Soil nematode fauna of afforested mine sites: genera distribution, trophic structure and functional guilds. Appl Soil Ecol 22:113–126
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Huhta V, Sulkava P, Viberg K (1998) Interactions between enchytraeid (Cognettia spagnetorum), microarthropod and nematode populations in forest soil at different moistures. Appl Soil Ecol 9:53–58
Hunt HW, Wall DH, DeCrapeo NM, Brenner JS (2001) A model for nematode locomotion in soil. Nematology 3:705–716
Hyvönen R, Persson T (1996) Effects of fungivorous and predatory arthropods on nematodes and tardigrades in microcosms with coniferous forest soil. Biol Fertil Soils 21:121–127
Hyvönen R, Andersson S, Clarholm M, Persson T (1994) Effects of lumbricids and enchytraeids on nematodes in limed and unlimed coniferous mor humus. Biol Fertil Soils 17:201–205
Ilieva-Makulec K, Makulec G (2002) Effect of the earthworm Lumbricus rubellus on the nematode community in a peat meadow soil. Eur J Soil Biol 38:59–62
Jeschke JM, Tollrian R (2000) Density-dependent effects of prey defences. Oecologia 123:391–396
Jeschke JM, Kopp M, Tollrian R (2004) Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol Rev 79:337–349
Keeling MJ, Wilson BH, Pacala SW (2000) Reinterpreting space, time lags, and functional responses in ecological models. Science 290:1758–1764
Khan Z, Bilgrami AL, Jairajpuri MS (1995) A comparative study on the predation by Allodorylaimus americanus and Discolaimus silvicolus (Nematoda: Dorylaimida) on different species of plant parasitic nematodes. Fundam Appl Nematol 18:99–108
Koehler HH (1997) Mesostigmata (Gamasina, Uropodina), efficient predators in agroecosystems. Agric Ecosyst Environ 62:105–117
Laakso J, Setälä H (1999) Population- and ecosystem-level effects of predation on microbial-feeding nematodes. Oecologia 120:279–286
Manly BFJ, Jamieson CD (1999) Functional response and parallel curve analysis. Oikos 85:523–528
Martikainen E, Huhta V (1990) Interactions between nematodes and predatory mites in raw humus soil: a microcosm experiment. Rev Ecol Biol Sol 27:13–20
McKee MH, Wrona FJ, Scrimgeour GJ, Culp JM (1997) Importance of consumptive and non-consumptive prey mortality in a coupled predator–prey system. Freshw Biol 38:193–201
Mikola J, Setälä H (1998a) No evidence of trophic cascades in an experimental microbial-based soil food web. Ecology 79:153–164
Mikola J, Setälä H (1998b) Productivity and trophic-level biomasses in a microbial-based soil food web. Oikos 82:158–168
Mikola J, Sulkava P (2001) Responses of microbial-feeding nematodes to organic matter distribution and predation in experimental soil habitat. Soil Biol Biochem 33:811–817
Moens T, Verbeeck L, Vines M (1999) Feeding biology of a predatory and a facultatively predatory nematode (Enoploides longispiculosus and Adoncholaimus fuscus). Mar Biol 134:585–593
Moens T, Herman P, Verbeeck L, Steyaert M, Vincx M (2000) Predation rates and prey selectivity in two predacious estuarine nematode species. Mar Ecol Prog Ser 205:185–193
Moore JC, De Ruiter PC (1997) Compartmentalisation of resource utilisation within soil ecosystems. In: Gange AC, Brown VK (eds) Multitrophic interactions in terrestrial systems. Blackwell Science, Oxford, pp 375–393
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Murphy IW, Doncaster CC (1957) A culture method for soil meiofauna and its application to the study of nematode predators. Nematologica 2:202–214
Nelson DR (2002) Current status of the Tardigrada: evolution and ecology. Integr Comp Biol 42:652–659
Nelson DR, Marley NJ (2000) The biology and ecology of lotic Tardigrada. Freshw Biol 44:93–108
Nelson DR, McInnes SJ (2002) Tardigrada. In: Rundle SD, Robertson AL, Schmid-Araya JM (eds) Freshwater meiofauna: biology and ecology. Backhuys Publishers, Leiden, Netherlands, pp 177–215
Nicholas WL (1975) The biology of free-living nematodes. Clarendon Press, Oxford
Parmelee RW (1995) Soil fauna: linking different levels of the ecological hierarchy. In: Jones CG, Lawton JK (eds) Linking species and ecosystems. Chapman and Hall, New York, pp 107–117
Pastorok RA (1981) Prey vulnerability and size selection by Chaoborus larvae. Ecology 62:1311–1324
Philips DA, Ferris H, Cook DR, Strong DR (2003) Molecular control points in rhizosphere food webs. Ecology 84:816–826
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383
Sayre RM (1969) A method of culturing a predacious tardigrade on the nematode Panagrellus redivivus. Trans Am Microsc Soc 88:266–274
Scheu S (2002) The soil food web: structure and perspectives. Eur J Soil Biol 38:11–20
Schmid-Araya JM, Schmid PE (2000) Trophic relationships: integrating meiofauna into a realistic benthic food web. Freshw Biol 44:149–163
Sih A (1987) Prey refuges and predator–prey stability. Theor Popul Biol 31:1–12
Small RW (1987) A review of the prey of predatory soil nematodes. Pedobiologia 30:179–206
Small RW, Grootaert P (1983) Observations on the predation abilities of some soil dwelling predatory nematodes. Nematol 29:109–118
Sohlenius B (1979) A carbon budget for nematodes, rotifers and tardigrades in a Swedish coniferous forest soil. Holarct Ecol 2:30–40
Sohlenius B (1980) Abundance, biomass and contribution to energy flow by soil nematodes in terrestrial ecosystems. Oikos 34:186–194
Traunspurger W (2002) Nematoda. In: Rundle SD, Robertson AL, Schmid-Araya JM (eds) Freshwater meiofauna: biology and ecology. Backhuys Publishers, Leiden, Netherlands, pp 63–104
Traunspurger W, Bergtold M, Goedkopp W (1997) The effects of nematodes on bacterial activity and abundance in a freshwater sediment. Oecologia 112:118–122
Wahlström E, Persson L, Diehl S, Byström P (2000) Size-dependent foraging efficiency, cannibalism and zooplankton community structure. Oecologia 123:138–148
Wallace HR (1958) Movement of eelworms. Ann Appl Biol 46:662–668
Walter DE, Hudgens RA, Freckman DW (1986) Consumption of nematodes by fungivorous mites, Tyrophagus spp (Acarina: Astigmata: Acaridae). Oecologia 70:357–361
Wardle DA (1995) Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Adv Ecol Res 26:105–185
Wasilewska L (2000) The effect of macroarthropods patrolling soil surface on soil nematodes: a field experiment in a mown meadow. Pol J Ecol 48:327–338
Yeates GW (1969) Predation by Mononchoides potohikus (Nematoda: Diplogateridae) in laboratory culture. Nematologica 15:1–9
Yeates GW (1987) Nematode feeding and activity: the importance of development stages. Biol Fertil Soils 3:143–146
Yeates GW, Foissner W (1995) Testate amoebae as predators of nematodes. Biol Fertil Soils 20:1–7
Yeates GW, Wardle DA (1996) Nematodes as predators and prey: relationships to biological control and soil processes. Pedobiologia 40:43–50
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25:315–331
Yeates GW, Dando JL, Shepherd TG (2002) Pressure plate studies to determine how moisture affects access of bacterial-feeding nematodes to food in soil. Eur J Soil Sci 53:355–365
Acknowledgements
We thank Hartmut Greven and Jonathan M. Jeschke who commented on earlier versions of this paper. The experiments comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hohberg, K., Traunspurger, W. Predator–prey interaction in soil food web: functional response, size-dependent foraging efficiency, and the influence of soil texture. Biol Fertil Soils 41, 419–427 (2005). https://doi.org/10.1007/s00374-005-0852-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0852-9