Abstract
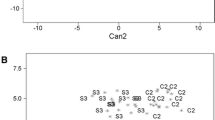
Avian yolk fatty acids (FA) composition is influenced by two main factors: maternal diet and genetic factors that regulate FA metabolism. However, due to embryonic developmental requirements, yolk FA are thought to be physiologically constrained and less useful for dietary and trophic studies. We assessed the relative contributions of diet and physiological constraints in determining the yolk FA composition of a marine bird, the gentoo penguin (Pygoscelis papua) by comparing FA signatures of yolks and prey between a captive, controlled- feeding experiment and a wild population. Captive and wild yolk FA signatures differed even though both groups’ yolk lipids were composed primarily of three FA (16:0, 18:0 and 18:1n-9). Differences were due to FA occurring in relatively low abundance, but which mirrored differences in the FA composition of diets. However, yolk FA signatures were correlated across three penguin species suggesting that common developmental constraints can be relatively more important than species-specific differences in diet or egg-laying physiology. While yolk FA are constrained, several minor components of yolk FA are reflective of diets and the calibration coefficients resulting from this study have the potential to be incorporated into predictive models and allow for quantitative dietary and trophic studies using FA analysis of penguin egg yolks.



Similar content being viewed by others
References
Anderson GJ, Connor WE, Corliss JD, Lin DS (1989) Rapid modulation of the n-3 docosahexaenoic acid levels in the brain and retina of the newly hatched chick. J Lipid Res 30:433–441
Astheimer LB, Grau CR (1990) A comparison of yolk growth-rates in seabird eggs. Ibis 132:380–394
Barrett RT, Camphuysen K, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Huppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691
Barton NWH, Fox NC, Surai PF, Speake BK (2002) Vitamins E and A, carotenoids, and fatty acids of the raptor egg yolk. J Raptor Res 36:33–38
Boyd I, Wanless S, Camphuysen CJ (eds) (2006) Top predators in marine ecosystems: their role in monitoring and management. Cambridge University Press, Cambridge
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Bremer J, Norum KR (1982) Metabolism of very long-chain monounsaturated fatty-acids (22:1) and the adaptation to their presence in the diet. J Lipid Res 23:243–256
Brown DJ, Boyd IL, Cripps GC, Butler PJ (1999) Fatty acid signature analysis from the milk of Antarctic fur seals and Southern elephant seals from South Georgia: implications for diet determination. Mar Ecol Prog Ser 187:251–263
Budge SM, Iverson SJ, Koopman HN (2006) Studying trophic ecology in marine ecosystems using fatty acids: A primer on analysis and interpretation. Mar Mammal Sci 22:759–801
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER, vers. 6: user manual/tutorial, PRIMER-E, Plymouth, UK
Clarke KR, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Connan M, Mayzaud P, Duhamel G, Bonnevie BT, Cherel Y (2010) Fatty acid signature analysis documents the diet of five myctophid fish from the Southern Ocean. Mar Biol 157:2303–2316
Cooper MH, Iverson SJ, Rouvinen-Watt K (2006) Metabolism of dietary cetoleic acid (22: 1n–11) in mink (Mustela vison) and gray seals (Halichoerus grypus) studied using radiolabeled fatty acids. Physiol Biochem Zool 79:820–829
Decrock F, Groscolas R, McCartney RJ, Speake BK (2001) Transfer of n-3 and n-6 polyunsaturated fatty acids from yolk to embryo during development of the king penguin. Am J Physiol-Reg I 280:R843–R853
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Groscolas R (1990) Metabolic adaptations to fasting in emperor and king penguins. In: Davis LS, Darby JT (eds) Penguin Biology. Academic Press, London, pp 269–296
Groscolas R, Frechard F, Decrock F, Speake BK (2003) Metabolic fate of yolk fatty acids in the developing king penguin embryo. Am J Physiol-Reg I 285:R850–R861
Hilditch TP, Williams PN (1964) The chemical constitution of natural fats. Chapman & Hall, London
Hintze J (2004) NCSS and PASS. Number Cruncher Statistical System, Kaysville, Utah
Iverson SJ (1993) Milk secretion in marine mammals in relation to foraging: can milk fatty acids predict diet? Sym Zool Soc Lond 66:263–291
Iverson SJ (2009) Tracing aquatic food webs using fatty acids: from qualitative indicators to quantitative determination. In: Kainz M, Brett MT, Arts MT (eds) Lipids in Aquatic Ecosystems. Springer, New York, pp 281–308
Iverson SJ, Lang SLC, Cooper MH (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36:1283–1287
Iverson SJ, Frost KJ, Lang SLC (2002) Fat content and fatty acid composition of forage fish and invertebrates in Prince William Sound, Alaska: factors contributing to among and within species variability. Mar Ecol Prog Ser 241:161–181
Iverson SJ, Field C, Bowen WD, Blanchard W (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol Monogr 74:211–235
Iverson SJ, Springer AM, Kitaysky AS (2007) Seabirds as indicators of food web structure and ecosystem variability: qualitative and quantitative diet analyses using fatty acids. Mar Ecol Prog Ser 352:235–244
Käkelä R, Furness RW, Kahle S, Becker PH, Käkelä A (2009) Fatty acid signatures in seabird plasma are a complex function of diet composition: a captive feeding trial with herring gulls. Funct Ecol 23:141–149
Käkelä R, Käkelä A, Martinez-Abrain A, Sarzo B, Louzao M, Gerique C, Villuendas E, Strandberg U, Furness RW, Oro D (2010) Fatty acid signature analysis confirms foraging resources of a globally endangered Mediterranean seabird species: calibration test and application to the wild. Mar Ecol Prog Ser 398:245–258
Koopman HN (2007) Phylogenetic, ecological, and ontogenetic factors influencing the biochemical structure of the blubber of odontocetes. Mar Biol 151:277–291
Lane HA, Westgate AJ, Koopman HK (2010) Ontogenetic and temporal variability in the fat content and fatty acid composition of Atlantic herring (Clupea harengus) from the Bay of Fundy, Canada. Fish Bull 109(1):113–122
Lipar JL, Ketterson ED, Nolan V (1999) Intraclutch variation in testosterone content of red-winged blackbird eggs. Auk 116:231–235
Miller AK, Karnovsky NJ, Trivelpiece WZ (2009) Flexible foraging strategies of gentoo penguins Pygoscelis papua over 5 years in the South Shetland Islands, Antarctica. Mar Biol 156:2527–2537
Miller AK, Kappes MA, Trivelpiece SG, Trivelpiece WZ (2010) Foraging-niche separation of breeding gentoo and chinstrap penguins, South Shetland Islands, Antarctica. Condor 112:683–695
Miyazaki M, Ntambi JM (2008) Fatty acid desaturation and chain elongation in mammals. In: Vance D, Vance J (eds) Biochemistry of lipids, lipoproteins and membranes. Elsevier, Amsterdam, pp 191–211
Offredo C, Ridoux V (1986) The diet of emperor penguins Aptenodytes forsteri in Adélie Land, Antarctica. Ibis 128:409–413
Oppel S, Powell AN, O’Brien DM (2010) King eiders use an income strategy for egg production: a case study for incorporating individual dietary variation into nutrient allocation research. Oecologia 164:1–12
Raclot T (2003) Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog Lipid Res 42:257–288
Raclot T, Groscolas R, Cherel Y (1998) Fatty acid evidence for the importance of myctophid fishes in the diet of king penguins, Aptenodytes patagonicus. Mar Biol 132:523–533
Ronconi RA, Koopman HN, McKinstry CAE, Wong SNP, Westgate AJ (2010) Inter-annual variability in diet of non-breeding pelagic seabirds Puffinus spp. at migratory staging areas: evidence from stable isotopes and fatty acids. Mar Ecol Prog Series 419:267–282
Speake BK, Murray AMB, Noble RC (1998) Transport and transformations of yolk lipids during development of the avian embryo. Prog Lipid Res 37:1–32
Speake BK, Decrock F, Surai PF, Groscolas R (1999a) Fatty acid composition of the adipose tissue and yolk lipids of a bird with a marine-based diet, the emperor penguin (Aptenodytes forsteri). Lipids 34:283–290
Speake BK, Surai PF, Noble RC, Beer JV, Wood NAR (1999b) Differences in egg lipid and antioxidant composition between wild and captive pheasants and geese. Comp Biochem Physiol B 124:101–107
Speake BK, Surai PF, Bortolotti GR (2002) Fatty acid profiles of yolk lipids of five species of wild ducks (Anatidae) differing in dietary preference. J Zool 257:533–538
Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP (1995) Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res 36:2471–2477
Surai PF, Speake BK (2008) The natural fatty acid compositions of eggs of wild birds and the consequences of domestication. In: Meester F, Watson RR (eds) Wild-Type food in health promotion and disease prevention: the columbus concept. Humana Press, Totowa, pp 121–137
Surai PF, Bortolotti GR, Fidgett AL, Blount JD, Speake BK (2001) Effects of piscivory on the fatty acid profiles and antioxidants of avian yolk: studies on eggs of the gannet, skua, pelican and cormorant. J Zool 255:305–312
Tierney M, Nichols PD, Wheatley KE, Hindell MA (2008) Blood fatty acids indicate inter- and intra-annual variation in the diet of Adelie penguins: comparison with stomach content and stable isotope analysis. J Exp Mar Biol Ecol 367:65–74
Trivelpiece WZ, Trivelpiece SG (1990) Courtship period of Adélie, gentoo and chinstrap penguins. In: Davis LS, Darby JT (eds) Penguin biology. Academic Press, London, pp 113–130
Ulberth F, Gabernig RG, Schrammel F (1999) Flame-ionization detector response to methyl, ethyl, propyl, and butyl esters of fatty acids. J Am Oil Chem Soc 76:263–266
Volkman NJ, Presler P, Trivelpiece W (1980) Diets of Pygoscelid penguins at King George Island, Antarctica. Condor 82:373–378
Wang SW, Hollmen TE, Iverson SJ (2010) Validating quantitative fatty acid signature analysis to estimate diets of spectacled and Steller’s eiders (Somateria fischeri and Polysticta stelleri). J Comp Physiol B 180:125–139
Williams CT, Buck CL (2010) Using fatty acids as dietary tracers in seabird trophic ecology: theory, application and limitations. J Ornithol 151:531–543
Williams CT, Iverson SJ, Buck CL (2009) The effects of diet and caloric restriction on adipose tissue fatty acid signatures of tufted puffin (Fratercula cirrhata) nestlings. J Comp Physiol B 179:711–720
Acknowledgments
We thank K. Vires, J. Beck, K. McGrath, T. Solberg and D. Rivard and the staff of the Scott Kingdoms of the Seas Aquarium for their helpful assistance with this study. Thank you to H. Lynch, R. Naveen, M. Rider, Oceanites Inc., Raytheon Polar Services and Linblad Expeditions for invaluable logistical support in Antarctica. We thank the US AMLR program and A. VanCise for access to krill samples and S. Wang for insights on sea-duck yolk FA. H. Lane and C. McKinstry provided for assistance with lipid extractions and statistical analyses. Thank you to A. Satake and two anonymous reviewers for helpful comments during the preparation of this manuscript. This research was funded by U.S. National Science Foundation (NSF) Office of Polar Programs (OPP) grants ANT-0125098 and ANT-0739575 to S. Emslie, and completed in accordance to animal use permits provided by Omaha’s Henry Doorly Zoo (HDZ#07-800) and NSF OPP (ACA 2006-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Polito, M.J., Koopman, H.N., Able, S. et al. Physiological constraints and the influence of diet on fatty acids in the yolk of gentoo penguins, Pygoscelis papua . J Comp Physiol B 182, 703–713 (2012). https://doi.org/10.1007/s00360-012-0649-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0649-8