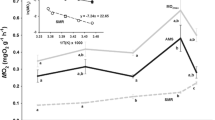
Abstract
Earlier work found cuttlefish (Sepia officinalis) ventilatory muscle tissue to progressively switch to an anaerobic mode of energy production at critical temperatures (T c) of 7.0 and 26.8°C. These findings suggested that oxygen availability limits thermal tolerance. The present study was designed to elucidate whether it is the ventilatory apparatus that sets critical temperature thresholds during acute thermal stress. Routine metabolic rate (rmr) rose exponentially between 11 and 23°C, while below (8°C) and above (26°C) this temperature range, rmr was significantly depressed. Ventilation frequency (f V) and mean mantle cavity pressure (MMP) followed an exponential relationship within the entire investigated temperature range (8–26°C). Oxygen extraction from the ventilatory current (EO2) decreased in a sigmoidal fashion with temperature, falling from > 90% at 8°C to 32% at 26°C. Consequently, ventilatory minute volume (MVV) increased by a factor of 20 from 7 to 150% body weight min−1 in the same temperature interval. Increases in MMP and MVV resulted in ventilatory muscle power output (P out) increasing by a factor of > 80 from 0.03 to 2.4 mW kg−1 animal. Nonetheless, costs for ventilatory mechanics remain below 1.5% rmr in the natural thermal window of the population (English Channel, 9–17°C), owing to very low MMPs of < 0.05 kPa driving the ventilatory stream, and may maximally rise to 8.6% rmr at 26°C. Model calculations suggest that the ventilatory system can maintain high arterial PO2 values of > 14 kPa over the entire temperature interval. We therefore conclude that the cuttlefish ventilation system is probably not limiting oxygen transfer during acute thermal stress. Depression of rmr, well before critical temperatures are being reached, is likely caused by circulatory capacity limitations and not by fatigue of ventilatory muscle fibres.




Similar content being viewed by others
References
Alexander RMcN (1970) Functional design in fishes. Hutchinson, London, 160 pp
Bartol IK (2001) Role of aerobic and anaerobic circular mantle muscle fibers in swimming squid: electromyography. Biol Bull 200:59–66
Bartol IK, Mann R, Patterson MR (2001) Aerobic respiratory costs of swimming in the negatively buoyant brief squid Lolliguncula brevis. J Exp Biol 204:3639–3653
Boal JG, Golden DK (1999) Distance chemoreception in the common cuttlefish Sepia officinalis (Mollusca, Cephalopoda). J Exp Mar Biol Ecol 235:307–317
Boal JG, Ni JN (1996) Ventilation rate of cuttlefish, Sepia officinalis, in response to visual stimuli. Veliger 38(4):342–347
Bone Q, Brown ER, Travers G (1994a) On the respiratory flow in the cuttlefish Sepia officinalis. J Exp Biol 194:153–165
Bone Q, Brown ER, Usher M (1994b) The structure and physiology of cephalopod muscle fibres. In: Abbot NJ, Williamson R, Maddock L (eds) Cephalopod neurobiology. Oxford University Press, Oxford, pp 301–329
Boucaud-Camou E, Boismery J (1991) The migrations of the cuttlefish (Sepia officinalis) in the English Channel. In: Boucaud-Camou E (ed) La Seiche, 1st international symposium on the cuttlefish Sepia. Centre of publications, Universite de Caen, pp 179–189
Burton RF (2002) Temperature and acid–base balance in ectothermic vertebrates: the imidazole alphastat hypothesis and beyond. J Exp Biol 205:3587–3600
Cameron JN, Cech JR (1970) Notes on the energy cost of gill ventilation in teleosts. Comp Biochem Physiol 34:447–455
Curtin NA, Woledge RC, Bone Q (2000) Energy storage by passive elastic structures in the mantle of Sepia officinalis. J Exp Biol 203:869–878
Denton EJ, Gilpin-Brown JB (1961) The buoyancy of the cuttlefish, Sepia officinalis (L.). J Mar Biol Assoc UK 41:319–342
Eno CN (1994) The morphometrics of cephalopod gills. J Mar Biol Assoc UK 74:687–706
Farrell AP (2002) Cardiorespiratory performance in salmonids during exercise at high temperature: insights into cardiovascular design limitations in fishes. Comp Biochem Physiol A 132:797–810
Gilmour KM (2001) The CO2/pH ventilatory drive in fish. Comp Biochem Physiol A 130:219–240
Heath AG, Hughes GM (1973) Cardiovascular and respiratory changes during heat stress in the rainbow trout (Salmo gairdneri). J Exp Biol 59:323–338
Houlihan DF, Innes AJ, Wells MJ, Wells J (1982) Oxygen consumption and blood gases of Octopus vulgaris in hypoxic conditions. J Comp Physiol 148:35–40
Houlihan DF, Agnisola C, Hamilton NM, Genoino TI (1987) Oxygen consumption of the isolated heart of octopus: effects of power output and hypoxia. J Exp Biol 131:137–157
Howell BJ, Gilbert DL (1976) pH–temperature dependence of the hemolymph of the squid, Loligo pealei. Comp Biochem Physiol A 55:287–289
Hughes GM (1973) Respiratory responses to hypoxia in fish. Am Zool 13:475–489
Jobling M (1982) A study of some factors affecting rates of oxygen consumption of plaice, Pleuronectes platessa L. J Fish Biol 20:501–516
Johansen K, Brix O, Kornerup S, Lykkeboe G (1982a) Factors affecting O2-uptake in the cuttlefish, Sepia officinalis. J Mar Biol Assoc UK 62:187–191
Johansen K, Beix O, Lykkeboe G (1982b) Blood gas transport in the cephalopod, Sepia officinalis. J Exp Biol 99:331–338
Jørgensen CB, Riisgård HU (1988) Gill pump characteristics of the soft clam Mya arenaria. Mar Biol 99:107–109
Lannig G, Bock C, Sartoris FJ, Pörtner HO (2004) Oxygen limitation of thermal tolerance in cod, Gadus morhua L. studied by magnetic resonance imaging (MRI) and on-line venous oxygen monitoring. Am J Physiol 287:R902–R910
Madan JJ, Wells MJ (1996) Cutaneous respiration in Octopus vulgaris. J Exp Biol 199:2477–2483
Mark FC, Bock C, Pörtner HO (2002) Oxygen-limited thermal tolerance in Antarctic fish investigated by MRI and 31P-MRS. Am J Physiol 283:R1254–R1262
Melzner F, Bock C, Pörtner HO (2006) Critical temperatures in the cephalopod Sepia officinalis investigated using in vivo 31P NMR spectroscopy. J Exp Biol 209:891–906
Messenger JB, Nixon M, Ryan KP (1985) Magnesiumchloride as an anaesthetic for cephalopods. Comp Biochem Physiol C 82:203–205
Milligan BJ, Curtin NA, Bone Q (1997) Contractile properties of obliquely striated muscle from the mantle of squid (Alloteuthis subulata) and cuttlefish (Sepia officinalis). J Exp Biol 200:2425–2436
Mislin H (1966) Über die Beziehungen zwischen Atmung und Kreislauf bei Cephalopoden (Sepia officinalis L.). Synchronregistrierung von Elektrokardiogramm (Ekg) und Atembewegungen am schwimmenden Tier. Verh Dtsch Zool Ges 175–181
O’Dor RK, Webber DM (1986) The constraints on cephalopods: why squid aren’t fish. Can J Zool 64:1591–1605
O’Dor RK, Webber DM (1991) Invertebrate athletes: trade-offs between transport efficiency and power density in cephalopod evolution. J Exp Biol 160:93–112
O’Dor RK, Wells MJ (1987) Energy and nutrient flow. In: Boyle PR (ed) Cephalopod life cycles. Comparative reviews, vol 2. Academic, London, pp 109–133
Packard A (1972) Cephalopods and fish: limits of convergence. Biol Rev 47:241–307
Perry SF, McKendry JE (2001) The relative roles of external and internal CO2 versus H+ in eliciting the cardiorespiratory responses of Salmo salar and Squalus acanthias to hypercarbia. J Exp Biol 204:3963–3971
Piiper J (1998) Branchial gas transfer models. Comp Biochem Physiol A 119:125–130
Pörtner HO (2002) Climate change and temperature dependent biogeography: systemic to molecular hierarchies of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761
Pörtner HO, Mark FC, Bock C (2004) Oxygen limited thermal tolerance in fish? Answers obtained by nuclear magnetic resonance techniques. Respir Physiol Neurobiol 141:243–260
Reeves RB (1972) An imidazole alphastat hypothesis for vertebrate acid–base regulation: tissue carbon dioxide content and body temperature in bullfrogs. Respir Physiol 14:219–236
Sartoris FJ, Bock C, Serendero I, Lannig G, Pörtner HO (2003) Temperature-dependent changes in energy metabolism, intracellular pH and blood oxygen tension in the Atlantic cod. J Fish Biol 62:1239–1253
Schumann D, Piiper J (1966) Der Sauerstoffbedarf der Atmung bei Fischen nach Messungen an der narkotisierten Schleie (Tinca tinca). Pflügers Archiv Eur J Physiol 288 (1):15–26
Seibel BA, Thuesen EV, Childress JJ, Gorodezky LA (1997) Decline in pelagic cephalopod metabolism with habitat depth reflects differences in locomotory efficiency. Biol Bull 192:262–278
Steffensen JF, Lomholt JP, Johansen K (1982) Gill ventilation and O2 extraction during graded hypoxia in two ecologically distinct species of flatfish, the flounder (Platichthys flesus) and the plaice (Pleuronectes platessa). Environ Biol Fish 2:157–163
Syme DA (1994) The efficiency of frog ventricular muscle. J Exp Biol 197:143–164
Terbeck S (2003) Der Einfluss von Kalium auf die Aktivität von Sepia officinalis. Diploma thesis, University of Münster, 61 pp
Vogel S (1994) Life in moving fluids: the physical biology of flow. Princeton University Press, Princeton, 467 pp
Webber DM, O’Dor RK (1986) Monitoring the metabolic rate and activity of free-swimming squid with telemetered jet pressure. J Exp Biol 126:202–224
Wells MJ (1990) Oxygen extraction and jet propulsion in cephalopods. Can J Zool 68:815–824
Wells MJ, Clarke A (1996) The costs of living and reproducing for an individual cephalopod. Philos Trans R Soc Lond B 351:1083–1104
Wells MJ, Wells J (1982) Ventilatory currents in the mantle of cephalopods. J Exp Biol 99:315–330
Wells MJ, Wells J (1983) The circulatory response to acute hypoxia in Octopus. J Exp Biol 104:59–71
Wells MJ, Wells J (1985a) Ventilation frequencies and stroke volumes in acute hypoxia in Octopus. J Exp Biol 118:445–448
Wells MJ, Wells J (1985b) Ventilation and oxygen uptake by Nautilus. J Exp Biol 118:297–312
Wells MJ, Wells J (1986) Blood flow in acute hypoxia in a cephalopod. J Exp Biol 122:345–353
Wells MJ, Wells J (1991) Is Sepia really an octopus? In: Boucaud-Camou E (ed) La Seiche, 1st international symposium on the cuttlefish Sepia. Centre de publications, Universite de Caen, pp 77–92
Wells MJ, Wells J (1995) The control of ventilatory and cardiac responses to changes in ambient oxygen tension and oxygen demand in octopus. J Exp Biol 198:1717–1727
Wells MJ, Hanlon RT, Lee PG, DiMarco FP (1988) Respiratory and cardiac performance in Lolliguncula brevis (Cephalopoda, Myopsida): the effects of activity, temperature and hypoxia. J Exp Biol 138:17–36
Zielinski S, Sartoris FJ, Pörtner HO (2001) Temperature effects on hemocyanin oxygen binding in an Antarctic cephalopod. Biol Bull 200:67–76
Acknowledgements
This study was carried out in support of the project “The cellular basis of standard and active metabolic rate in the free-ranging cephalopod, Sepia officinalis” (NER/A/S/2002/00812). The authors wish to thank Rolf Wittig and Timo Hirse for their excellent technical support, Raymond and Marie-Paule Chichery (Université de Caen) for providing cuttlefish eggs in 2002 and 2003 and all student helpers that were engaged in raising the animals in our lab.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Melzner, F., Bock, C. & Pörtner, H.O. Temperature-dependent oxygen extraction from the ventilatory current and the costs of ventilation in the cephalopod Sepia officinalis . J Comp Physiol B 176, 607–621 (2006). https://doi.org/10.1007/s00360-006-0084-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-006-0084-9