Abstract
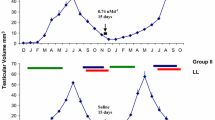
To explore the role of TH in the control of seasonality [i.e., photoperiodic testicular growth, photorefractoriness, and postnuptial (prebasic) molt] in American tree sparrows (Spizella arborea), we performed experiments in which THX males were simultaneously photostimulated and given TH replacement therapy. In the first experiment, equimolar concentrations (1X=1.3 nmol) of T4, T3, or GC-1, an iodine-free TRβ agonist, were administered s.c. daily during the first 21 days of photostimulation. Two additional THX groups received GC-1 at 0.1X or 10X, and THX and THI control groups received vehicle. In the second experiment, T4 or T3, alone or in combination with the deiodinase inhibitor IOP, was injected i.m. twice daily during the first 14 days of photostimulation. In both experiments, end points were testis length and molt score. In the first experiment, THI birds given vehicle and THX birds given T4 replacement therapy exhibited all three components of seasonality. THX birds given T3 or GC-1 (1X or 10X) showed a subdued photoperiodic testicular response, but they did not become photorefractory or initiate molt. THX birds that received 0.1X GC-1 or vehicle exhibited none of the components of seasonality. These data are consistent with the hypothesis that photoperiodic testicular growth, a vernal component of seasonality, is a TRβ-mediated response and suggest that T4 may activate TRβ more efficiently than does T3 or GC-1. By contrast, the failure both of T3 and of GC-1, but not of T4, to program photostimulated THX males for photorefractoriness and postnuptial molt suggests that autumnal components of seasonality may be TRα-mediated responses solely to T4. In the second experiment, IOP administered alone had no significant impact on seasonality. THX birds that received T4 with or without IOP showed all components of seasonality, whereas birds that received T3 with or without IOP showed only photoperiodic testicular growth. These results challenge the widely held view that T4 is merely a prohormone for T3 and support the emerging view that T4 has intrinsic hormonal activity. Because IOP augmented the photoperiodic testicular response in T3-treated THX birds, T3 may act either independently or co-dependently with T4 in programming vernal seasonal events.




Similar content being viewed by others
Abbreviations
- TH :
-
thyroid hormone
- THX :
-
thyroidectomized
- THI :
-
thyroid-intact
- T4 :
-
thyroxine (3,5,3′5′-tetraiodo-l-thyronine)
- T3 :
-
triiodothyronine (3,5,3′-triiodo-l-thyronine)
- rT3 :
-
reverse triiodothyronine (3,3′,5′-triiodo-l-thyronine)
- T2 :
-
diiodothyronine (3,3′-diiodo-l-thyronine)
- GC-1 :
-
3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)-phenoxy acetic acid
- TR :
-
thyroid receptor
- IOP :
-
iopanoic acid
- s.c. :
-
subcutaneously
- i.m. :
-
intramuscularly
- i.c.v. :
-
intracerebroventricular
- RXR :
-
retinoid X receptor
References
Anderson GW (2001) Thyroid hormones and the brain. Front Neuroendocrinol 22:1–17
Andersson ML, Nordström K, Demczuk S, Harbers M, Vennström B (1992) Thyroid hormone alters the DNA binding properties of chicken thyroid hormone receptors α and β. Nucleic Acids Res 20:4803–4810
Baxter JD, Dillmann WH, West BL, Huber R, Furlow JD, Fletterick RJ, Webb P, Apriletti JW, Scanlan TS (2001) Selective modulation of thyroid hormone receptor action. J Steroid Biochem Mol Biol 76:31–42
Baxter JD, Goede P, Apriletti JW, West BL, Feng W, Mellstrom K, Fletterick RJ, Wagner RL, Kushner PJ, Ribeiro RCJ, Webb P, Scanlan TS, Nilsson S (2002) Structure-based design and synthesis of a thyroid hormone receptor (TR) antagonist. Endocrinology 143:517–524
Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA (1997) The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinology 138:2989–2997
Bogazzi F, Bartalena L, Brogioni S, Burelli A, Grasso L, Dell’Unto E, Manetti L, Martino E (1997) l-thyroxine directly affects expression of thyroid hormone-sensitive genes: regulatory effect of RXRβ. Mol Cell Endocrinol 134:23–31
Dawson A, Thapliyal JP (2001) The thyroid and photoperiodism. In: Dawson A, Chaturvedi CM (eds) Avian endocrinology. Narosa Publishing House, New Delhi, pp 141–151
de Jong M, Docter R, Van der Hoek H, Krenning E, Van der Heide D, Quero C, Plaisier P, Vos R, Hennemann G (1994) Different effects of amiodarone on transport of T4 and T3 into the perfused rat liver. Am J Physiol Endocrinol Metab 266:E44–E49
Escobar-Morreale HF, Escobar del Rey F, Obregón MJ, Morreale de Escobar G (1996) Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 137:2490–2502
Everts ME, Docter R, Moerings EP, van Koetsveld PM, Visser TJ, de Jong M, Krenning EP, Hennemann G (1994) Uptake of thyroxine in cultured anterior pituitary cells of euthyroid rats. Endocrinology 134:2490–2497
Everts ME, de Jong M, Lim CF, Docter R, Krenning EP, Visser TJ, Hennemann G (1996) Different regulation of thyroid hormone transport in liver and pituitary: its possible role in the maintenance of low T3 production during nonthyroidal illness and fasting in man. Thyroid 6:359–368
Forrest D, Hallbook F, Persson H, Vennström B (1991) Distinct functions for thyroid hormone receptors alpha and beta in brain development indicated by differential expression of receptor genes. EMBO J 10:269–275
Göthe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennström B, Forrest D (1999) Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary–thyroid axis, growth, and bone maturation. Genes Dev 13:1329–1341
Hashimoto K, Curty FH, Borges PP, Lee CE, Abel ED, Elmquist JK, Cohen RN, Wondisford FE (2001) An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA 98:3998–4003
Hennemann G, Everts ME, de Jong M, Lim CF, Krenning EP, Docter R (1998) The significance of plasma membrane transport in the bioavailability of thyroid hormone. Clin Endocrinol (Oxf) 48:1–8
Ichikawa K, Miyamoto T, Kakizawa T, Suzuki S, Kaneko A, Mori J, Hara M, Kumagai M, Takeda T, Hashizume K (2000) Mechanism of liver-selective thyromimetic activity of SK&F L-94901: evidence for the presence of a cell-type-specific nuclear iodothyronine transport process. J Endocrinol 165:391–397
Jorgensen EC (1978) Thyroid hormones and analogs. II. Structure-activity relationships. In: Li CH (ed) Hormonal proteins and peptides. Academic Press, New York, pp 107–204
Moreno M, Lombardi A, Beneduce L, Silvestri E, Pinna G, Goglia F, Lanni A (2002) Are the effects of T3 on resting metabolic rate in euthyroid rats entirely caused by T3 itself? Endocrinology 143:504–510
Morte B, Manzano J, Scanlan T, Vennström B, Bernal J (2002) Deletion of the thyroid hormone receptor prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989
Newton I (1966) The moult of the bullfinch Pyrrhula pyrrhula. Ibis 108:41–67
Oppenheimer JH (1999) Evolving concepts of thyroid hormone action. Biochimie 81:539–543
Pant K, Chandola-Saklani A (1993a) Effects of thyroxine on avian moulting may not involve prior conversion to tri-iodothyronine. J Endocrinol 137:265–270
Pant K, Chandola-Saklani A (1993b) A role for thyroid hormones in the development of premigratory disposition in redheaded bunting, Emberiza bruniceps. J Comp Physiol B 163:389–394
Pant K, Chandola-Saklani A (1995) T3 fails to mimic certain effects of T4 in munia birds: physiological implications for seasonal timing. Comp Biochem Physiol C 111:157–164
Prendergast BJ, Mosinger B, Jr., Kolattukudy PE,Nelson RJ (2002) Hypothalamic gene expression in reproductively photoresponsive and photorefractory Siberian hamsters. Proc Natl Acad Sci USA 99:16291–16296
Quack M, Carlberg C (2001) Single thyroid hormone receptor monomers are competent for co-activator-mediated transactivation. Biochem J 360:387–393
Reinert BD, Wilson FE (1996) The thyroid and the hypothalamus–pituitary–ovarian axis in American tree sparrows (Spizella arborea). Gen Comp Endocrinol 103:60–70
Reinert BD, Wilson FE (1997) Effects of thyroxine (T4) or triiodothyronine (T3) replacement therapy on the programming of seasonal reproduction and postnuptial molt in thyroidectomized male American tree sparrows (Spizella arborea) exposed to long days. J Exp Zool 279:367–376
Ribeiro RCJ, Apriletti, JW, Wagner RL, Feng W, Kushner PJ, Nilsson S, Scanlan TS, West BL, Fletterick RJ, Baxter JD (1998) X-ray crystallographic and functional studies of thyroid hormone receptor. J Steroid Biochem Mol Biol 65:133–141
Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA (2001) Thyroid hormone-sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest 108:97–105
Rosic MA, Pantovic,SB, Lucic A.P, Ribarac-Stepic N, Andjelkovic IZ (2001) Kinetics of thyroxine (T4) and triiodothyronine (T3) transport in the isolated rat heart. Exp Physiol 86(1):13–18
Rudas P, Bartha T (1993) Thyroxine and triiodothyronine uptake by the brain of chickens. Acta Vet Hung 41:395–408
Rudas P, Pethes G (1989) Adaptation of 5′-deiodination to hypothyroid conditions following surgical and/or radiothyroidectomy in chickens. Acta Vet Hung 37:227–239
Rudas P, Bartha T, Frenyó LV (1993) Thyroid hormone deiodination in the brain of young chickens acutely adapts to changes in thyroid status. Acta Vet Hung 41:381–393
Runfeldt S, Wingfield JC (1985) Experimentally prolonged sexual activity in female sparrows delays termination of reproductive activity in their untreated mates. Anim Behav 33:403–410
Scanlan TS, Yoshihara HA, Nguyen NH, Chiellini G (2001) Selective thyromimetics: tissue-selective thyroid hormone analogs. Curr Opin Drug Discov Dev 4:614–622
Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH (2000) The thyroid hormone receptor β-selective agonist GC-1 differentially affects plasma level and cardiac activity. Endocrinology 141:3057–3064
Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ (2001) Hormone selectivity in thyroid hormone receptors. Mol Endocrinol 15:398–410
Wilson FE (1997) Photoperiodism in American tree sparrows: role of the thyroid gland. In: Harvey S, Etches RJ (eds) Perspectives in avian endocrinology. The Endocrine Society Press, Bristol, pp 159–169
Wilson FE (2001) A test of the hypothesis that T3 is the “seasonality” thyroid hormone in American tree sparrows (Spizella arborea): intracerebroventricular infusion of iopanoic acid, an inhibitor of T3 synthesis and degradation. J Comp Physiol B 171:113–119
Wilson FE, Reinert BD (1993) The thyroid and photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea). J Comp Physiol B 163:563–573
Wilson FE, Reinert BD (1995) A one-time injection of thyroxine programmed seasonal reproduction and postnuptial molt in chronically thyroidectomized male American tree sparrows Spizella arborea. J Avian Biol 26:225–233
Wilson FE, Reinert BD (1996) The timing of thyroid-dependent programming in seasonally breeding male American tree sparrows (Spizella arborea). Gen Comp Endocrinol 103:82–92
Wilson FE, Reinert BD (1999) Long days and thyroxine program American tree sparrows for seasonality: evidence for temporal flexibility of the breeding season of euthyroid females. Gen Comp Endocrinol 113:136–145
Wilson FE, Reinert BD (2000) Thyroid hormone acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) for vernal and autumnal components of seasonality. J Neuroendocrinol 12:87–95
Yiannakouris N, Valcana T (1998) Differences in pattern of release of triiodothyronine (T3) and tetraiodothyronine (T4) associated receptors from chromatin by micrococcal nuclease. Horm Metab Res 30:7–11
Yoshimasa Y, Hamada S (1983) Thyroxine action on the rat liver nuclear thyroid-hormone receptors. Biochem J 210:331–337
Acknowledgements
This investigation was supported in part by a grant (IBN-9982281) from the National Science Foundation to F.E.W. and in part by a grant (DK-52798) from the National Institutes of Health to T.S.S.
Author information
Authors and Affiliations
Corresponding author
Additional information
A preliminary report was presented at ENDO 2001 Denver (M.K. Mishra, T.S. Scanlan, G. Chiellini, and F.E. Wilson, Abstract P2-517)
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Mishra, M.K., Wilson, F.E., Scanlan, T.S. et al. Thyroid hormone-dependent seasonality in American tree sparrows (Spizella arborea): effects of GC-1, a thyroid receptor β-selective agonist, and of iopanoic acid, a deiodinase inhibitor. J Comp Physiol B 174, 471–479 (2004). https://doi.org/10.1007/s00360-004-0433-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-004-0433-5