Abstract
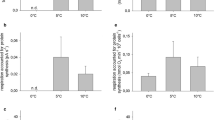
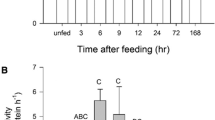
The translational system was isolated from the gills of the Antarctic scallop Adamussium colbecki (Smith) and the European scallop Aequipecten opercularis (Linnaeus) for in vitro protein synthesis capacities (μg protein mg FW−1 day−1) and the translational capacities of RNA (kRNA in vitro mg protein mg RNA−1 day−1). In vitro protein synthesis capacity in the cold-adapted pectinid at 0 °C was similar to the one found in the temperate scallop at 25 °C. These findings might reflect cold compensated rates in Adamussium colbecki, partly explainable by high tissue levels of RNA. Cold-compensated in vitro protein synthesis capacities may further result from increments in the translational capacity of RNA. The thermal sensitivity of the translation machinery was slightly different in the two species, with significantly lower levels of Arrhenius activation energies Ea and Q10 in Adamussium colbecki in the temperature range 0–15 °C. Reduced protein synthesis and translational capacities were found in vitro in gills of long-term aquarium-maintained Adamussium colbecki and were accounted for by a loss of protein synthesis machinery, i.e. a reduction in RNA levels, as well as a decrease in the amount of protein synthesized per milligram of RNA (RNA translational capacity, kRNA in vitro). Such changes may involve food uptake or mirror metabolic depression strategies, like those occurring during winter. Consequences of high in vitro RNA translational capacities found in the permanently cold-adapted species are discussed in the context of seasonal food availability and growth rates at high latitudes.




Similar content being viewed by others
Abbreviations
- DPM :
-
disintegrations per minute
- DTT :
-
dithiothreitol
- E a :
-
Arrhenius activation energy
- k s :
-
fractional protein synthesis rate
- k RNA in vivo :
-
translational efficiency
- k RNA in vitro :
-
translational capacity
- PCA :
-
perchloric acid
- Phe :
-
phenylalanine
- PLA :
-
phospho-L-arginine
- PSU :
-
practical salinity units
- RNAse :
-
ribonuclease
- TCA :
-
trichloroacetic acid
References
Ashford AJ, Pain VM (1986) Effect of diabetes on the rates of synthesis and degradation of ribosomes in rat muscle and liver in vivo. J Biol Chem 261:4059–4065
Bailey DM, Peck LS, Bock C, Pörtner HO (2003) High energy phosphate metabolism during exercise and recovery in temperate and Antarctic scallops–an in vivo 31P-NMR study. Physiol Biochem Zool (In press)
Brey T, Clarke A (1993) Population dynamics of marine benthic invertebrates in Antarctic and subantarctic environments: are there unique adaptations. Ant Sci 5:253–266
Canapa A, Barucca M, Marinelli A, Olmo E (2000) Molecular data from the 16S rRNA gene for the phylogeny of Pectinidae (Mollusca: Bivalvia). J Mol Evol 50:93–97
Clarke A, Leakey RJG (1996) The seasonal cycle of phytoplankton, macronutrients, and the microbial community in a nearshore Antarctic marine ecosystem. Limnol Oceanogr 41:1281–1294
Foster AR, Houlihan DF, Hall SJ, Burren LJ (1992) The effects of temperature acclimation on protein synthesis rates and nucleic acid content of juvenile cod (Gadus morhua L.). Can J Zool 70:2095–2102
Fraser KP, Clarke A, Peck LS (2002) Low-temperature protein metabolism: seasonal changes in protein synthesis and RNA dynamics in the Antarctic limpet Nacella concinna Strebel (1908). J Exp Biol 205:3077–3086
Haschemeyer AE (1983) A comparative study of protein synthesis in nototheniids and icefish at Palmer Station, Antarctica. Comp Biochem Physiol B 76:541–543
Haschemeyer AE, Persell R, Smith MA (1979) Effect of temperature on protein synthesis in fish of the Galapagos and Perlas Islands. Comp Biochem Physiol B 64:91–95
Haschemeyer AEV, Williams RC Jr (1982) Temperature dependency of cell-free protein synthetic systems from Antarctic fish. Mar Biol Lett 3:81–88
Hawkins AJS (1991) Protein turnover: a functional appraisal. Funct Ecol 5:222–233
Heilmayer O, Brey T (2003) Saving by freezing? Metabolic rates of Adamussium colbecki in a latitudinal context. Mar Biol 143:477–484
Heilmayer O, Brey T, Storch D, Mackensen A, Arntz WE (2004) Population dynamics and metabolism of Aequipecten opercularis (L.) from the western English Channel (Roscoff, France). Neth J Sea Res (In press)
Hershey JWB (1991) Translational control in mammalian cells. Annu Rev Biochem 60:717–755
Hofmann GE, Hand SC (1994) Global arrest of translation during invertebrate quiescence. Proc Natl Acad Sci USA 91:8492–8496
Houlihan DF (1991) Protein turnover in ectotherms and its relationships to energetics. In: Gilles R (ed) Advances in comparative and environmental physiology, vol 7. Springer, Berlin Heidelberg New York, pp 1–43
Houlihan DF, McMillan DN, Laurent P (1986) Growth rates, protein synthesis, and protein degradation rates in rainbow trout: effects of body size. Physiol Zool 59:482–493
Houlihan DF, Hall SJ, Gray C (1989) Effects of ration on protein turnover in cod. Aquaculture 79:103–110
Houlihan DF, McMillan DN, Agnisola C, Trara Genoino I, Foti L (1990a) Protein synthesis and growth in Octopus vulgaris. Mar Biol 106:251–259
Houlihan DF, Waring CP, Mathers E, Gray C (1990b) Protein synthesis and oxygen consumption of the shore crab Carcinus maenas after a meal. Physiol Zool 63:735–756
Kim DM, Swartz JR (2000) Prolonging cell-free protein synthesis by selective reagent additions. Biotechnol Prog 16:385–390
Lied E, Lie O, Lambertsen G (1985) Nutritional evaluation in fish by measurement of in vitro protein synthesis in white trunk muscle tissue. In: Conwey CB, Mackie AM, Bell JG (eds) Nutrition and feeding in fish. Academic Press, London, pp 169–176
Lyndon AR, Houlihan DF (1998) Gill protein turnover: costs of adaptation. Comp Biochem Physiol A 119:27–34
Marsh AG, Maxson RE Jr, Manahan DT (2001) High macromolecular synthesis with low metabolic cost in Antarctic sea urchin embryos. Science 291:1950–1952
McMillan DN, Houlihan DF (1988) The effect of refeeding on tissue protein synthesis in rainbow trout. Physiol Zool 61:429–441
McMillan DN, Houlihan DF (1989) Short-term responses of protein synthesis to re-feeding in rainbow trout. Aquaculture 79:37–46
Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS (1973) Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241:204–205
Munro HN, Fleck A (1966) Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst 91:78–88
Peck LS (2002) Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol 25:31–40
Pörtner HO (2002) Physiological basis of temperature-dependent biogeography: trade-offs in muscle design and performance in polar ectotherms. J Exp Biol 205:2217–2230
Ray KM, Kumar GS, Janiyani K, Kannan K, Jagtap P, Basu MK, Shivaji S (1998) Adaptation to low temperature and regulation of gene expression in antartic psychrotrophic bacteria. J Biosci 23:423–435
Robertson RF, El-Haj AJ, Clarke A, Peck LS, Taylor EW (2001) The effects of temperature on metabolic rate and protein synthesis following a meal in the isopod Glyptonotus antarcticus Eights (1852). Polar Biol 24:677–686
Segel LA (1976) Incorporation of receptor kinetics into a model for bacterial chemotaxis. J Theor Biol 57:23–42
Smith MAK, Haschemeyer AEV (1980) Protein metabolism and cold adaptation in Antarctic fishes. Physiol Zool 53:373–382
Spirin AS, Baranov VI, Ryabova LA, Ovodov SY, Alakhov YB (1988) A continuous cell-free translation system capable of producing polypeptides in high yield. Science 242:1162–1164
Storch D, Pörtner HO (2003) The protein synthesis machinery operates at the same expense in eurythermal and cold stenothermal pectinids. Physiol Biochem Zool 76:76:28–40
Thomas T, Cavicchioli R (2002) Cold adaptation of archaeal elongation factor 2 (EF-2) proteins. Curr Protein Pept Sci 3:223–230
Walton GM, Gill GN (1976) Preferential regulation of protein synthesis initiation complex formation by purine nucleotides. Biochim Biophys Acta 447:11–19
Waterlow JC, Garlick PJ, Millward DJ (1978) The relationship between protein synthesis and cell growth. In: Waterlow JC, Garlick PJ, Millward DJ (eds) Protein turnover in mammalian tissues and in the whole body. Elsevier, Amsterdam, pp 538–557
Whiteley NM, Taylor EW, Haj AJ el (1996) A comparison of the metabolic cost of protein synthesis in stenothermal and eurythermal isopod crustaceans. Am J Physiol 271:R1295–R1303
Wollenberger AO, Ristau O, Schoffa G (1960) Eine einfache Technik der extrem schnellen Abkühlung grosser Gewebestücke. Eur J Physiol 270:399–412
Acknowledgements
We are grateful to the staff of the "Station Biologique de Roscoff" for providing Aequipecten opercularis. The work on Antarctic scallops was carried out as part of the Italian research programme "Progetto Nazionale Ricerche in Antartide" (PNRA). We would like to thank Riccardo Cattaneo Vietti and Chiara Chiantore for the invitation to their beautiful Italian base "Terra Nova Bay". The experiments conducted in this study comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Storch, D., Heilmayer, O., Hardewig, I. et al. In vitro protein synthesis capacities in a cold stenothermal and a temperate eurythermal pectinid. J Comp Physiol B 173, 611–620 (2003). https://doi.org/10.1007/s00360-003-0371-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-003-0371-7