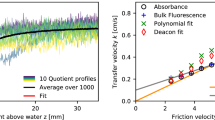
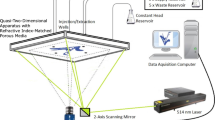
Abstract
A laser-induced fluorescence (LIF) technique is employed for visualizing a thin two-dimensional (2D) dissolved oxygen concentration field and measuring local oxygen concentration gradients near the surface of an oxygen bubble in water containing surfactant (Triton X-100, SigmaAldrich, St Louis, MO, USA)). The fluorescence of pyrene butyric acid (PBA) is induced by a planar pulse of nitrogen laser light. Oxygen transferring from the bubble to the deoxygenated water quenches the fluorescence of the PBA. Images of the fluorescence fields are captured by a UV-intensified CCD camera. The intensity of fluorescence quenching at each image pixel is used to measure dissolved oxygen concentration in a 2D field. Images of bubbles are obtained at 200 ppm, 100 ppm, and 50 ppm Triton X-100-containing water and in ultra clean deionized water. Higher surfactant concentrations decrease local and average concentration gradients of oxygen at the bubble surface. The ensemble means of dissolved oxygen concentration boundary layer thicknesses of 0.160 mm, 0.130 mm, and 0.072 mm, for the images of bubbles obtained at 200 ppm, 100 ppm, and 50 ppm Triton X-100-containing water, respectively. Local concentration boundary layer thickness increases from the top to the bottom along the bubble surface. A series of images of the bubble flow fields are analyzed to measure the oxygen concentration gradients in water in the presence of surfactant. The images captured in clean water are not fully resolvable because of their poor resolution. The formation of the attached wake in the fluorescence field images at the bottom of the bubbles in clean water tends to be promoted by increasing oblateness owing to the presence of surfactant at the surface.












Similar content being viewed by others
References
Azbel D (1981) Two-phase flows in chemical engineering. Cambridge University Press, Cambridge, pp 155–167
Clift R, Grace JR, Weber ME (1978) Bubbles, drops and particles. Academic Press, New York, pp 278–301
Danckwerts PV (1951 Significance of liquid-film coefficients in gas absorption. Ind Eng Chem 43:1460
Duke SR (1996) Air–water transfer at wavy interfaces. PhD dissertation, University of Illinois
Goodridge F, Robb ID (1965) Mechanism of interfacial resistance in gas absorption. IEC Fundamentals 4:49–55
Higbie R (1935) The rate of absorption of a pure gas into a still liquid during short periods of exposure. Trans Am Inst Chem Eng 31:365–368
Jähne B, Monahan EC (1995) Air–water gas transfer. In Third International Symposium on Air–water gas transfer. Aeon, Hanau, Germany, pp 339–369
Lewis WK, Whitman WG (1924) Principles of gas absorption. Ind Eng Chem 16:1215
Munsterer T (1996) LIF investigation of the mechanisms controlling air–water mass transfer at a free interface. PhD thesis, University of Heidelberg
Roy S, Duke SR (2000) Laser induced fluorescence measurements of dissolved oxygen concentration fields near air bubble surfaces. Rev Sci Instrum 71:3494–3502
Vaughan WM, Weber G (1970) Oxygen quenching of pyrenebutyric acid fluorescence in water: a dynamic probe of the microenvironment. Biochemistry 9:464–473
Acknowledgements
This work was partially supported by grants from the US Geological Survey Water Resources Research Program and the Auburn University Competitive Research Program.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Roy, S., Duke, S.R. Visualization of oxygen concentration fields and measurement of concentration gradients at bubble surfaces in surfactant-contaminated water. Exp Fluids 36, 654–662 (2004). https://doi.org/10.1007/s00348-003-0771-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00348-003-0771-1