Abstract
Purpose
To conduct a scoping review of the existing literature and recent developments on prostatic stents for the treatment of benign prostatic hyperplasia (BPH).
Methods
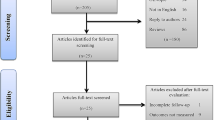
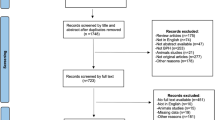
A comprehensive search was performed on Embase, MEDLINE, and Web of Science to identify English literature on prostatic stents for the treatment of BPH. Additional studies and upcoming devices were identified through grey literature search and expert consultation. Study characteristics and stent information were extracted and tabulated narratively.
Results
Of the 1171 search results, 64 studies were included in this review. iTiND was the prostatic stent with the most long-term evidence. iTiND is a safe and effective minimally invasive treatment for BPH that preserves sexual function. Adverse events are mild and transitory. Emerging stents (e.g. Zenflow, Butterfly, Urocross, and Exime) had 7/64 eligible studies, where no studies had long-term follow-up. These newer stents show promising results for quality of life and BPH symptom management; however, long-term monitoring and head-to-head comparisons are needed.
Conclusion
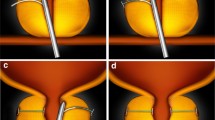
Over the last 50 years, prostatic stents have evolved and demonstrated improved clinical efficacy. iTiND provides a safe and effective outpatient treatment of LUTS secondary to BPH preserving erectile and ejaculatory function. Emerging prostatic stents are a promising, effective, and safe intervention in well-selected patients interested in its benefits.
Similar content being viewed by others
Data Availability
Scoping review data is available in the Supplementary Material.
References
Parsons JK (2010) Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep 5:212–218. https://doi.org/10.1007/s11884-010-0067-2
Garcia-Argibay M, Hiyoshi A, Fall K, Montgomery S (2022) Association of 5α-reductase inhibitors with dementia, depression, and suicide. JAMA Netw Open 5:e2248135. https://doi.org/10.1001/jamanetworkopen.2022.48135
La Torre A, Palleria C, Tamanini I et al (2021) Sexual dysfunctions related to drugs used in the management of lower urinary tract symptoms due to benign prostatic hyperplasia: a narrative review on α-blockers and 5-alpha reductase inhibitors. Uro 1:82–98. https://doi.org/10.3390/uro1030012
Sountoulides P, Karatzas A, Gravas S (2019) Current and emerging mechanical minimally invasive therapies for benign prostatic obstruction. Ther Adv Urol 11:1756287219828971. https://doi.org/10.1177/1756287219828971
Rassweiler J, Teber D, Kuntz R, Hofmann R (2006) Complications of transurethral resection of the prostate (TURP)–incidence, management, and prevention. Eur Urol 50:969–979. https://doi.org/10.1016/j.eururo.2005.12.042. (discussion 980)
Elterman D, Gao B, Zorn KC et al (2021) How i do it: temporarily implanted nitinol device (iTind). Can J Urol 28:10788–10793
Bouhadana D, Nguyen D-D, Zorn KC et al (2020) Patient perspectives on benign prostatic hyperplasia surgery: a focus on sexual health. J Sex Med 17:2108–2112. https://doi.org/10.1016/j.jsxm.2020.07.006
Milroy E, Allen A (1996) Long-term results of urolume urethral stent for recurrent urethral strictures. J Urol 155:904–908
Oesterling JE (1994) The UroLume endoprosthesis: a summary of the European and North American experience. Prog Clin Biol Res 386:561–575
De Vocht TF, van Venrooij GEPM, Boon TA (2003) Self-expanding stent insertion for urethral strictures: a 10-year follow-up. BJU Int 91:627–630. https://doi.org/10.1046/j.1464-410x.2003.04200.x
Eaton Turner E, Jenks M, McCool R et al (2018) The Memokath-051 stent for the treatment of ureteric obstruction: a NICE medical technology guidance. Appl Health Econ Health Policy 16:445–464. https://doi.org/10.1007/s40258-018-0389-3
Dorkin T, Crundwell M, Speakman M (2017) Memokath-028, 044 and 045 stents for urethral obstruction. In: National Institute for Health and Care Excellence. https://www.nice.org.uk/advice/mib123/resources/memokath028-044-and-045-stents-for-urethral-obstruction-pdf-2285963333552581. Accessed 28 Dec 2022
Song PH, Kim YU, Choi JY et al (2015) Efficacy of thermo-expandable intra-prostatic stent (memokathTM028) as an alternative approach for benign prostatic hyperplasia patients with significant comorbidities: comparison with transurethral resection of the prostate. J Urol 193:e23–e24
Bozkurt IH, Yalcinkaya F, Sertcelik MN et al (2013) A good alternative to indwelling catheter owing to benign prostate hyperplasia in elderly: memotherm prostatic stent. Urology 82:1004–1007. https://doi.org/10.1016/j.urology.2013.07.004
Laaksovirta S, Talja M, Valimaa T et al (2001) Expansion and bioabsorption of the self-reinforced lactic and glycolic acid copolymer prostatic spiral stent. J Urol 166:919–922
Kotsar A, Isotalo T, Juuti H et al (2009) Biodegradable braided poly(lactic-co-glycolic acid) urethral stent combined with dutasteride in the treatment of acute urinary retention due to benign prostatic enlargement: a pilot study. BJU Int 103:626–629. https://doi.org/10.1111/j.1464-410X.2008.08111.x
Yildiz G, Bahouth Z, Halachmi S et al (2016) Allium TM TPS–a new prostatic stent for the treatment of patients with benign prostatic obstruction: the first report. J Endourol 30:319–322. https://doi.org/10.1089/end.2015.0593
Bahouth Z, Moskovitz B, Halachmi S, Nativ O (2017) Allium stents: a novel solution for the management of upper and lower urinary tract strictures. Rambam maimonides. Med J. https://doi.org/10.5041/RMMJ.10313
Pizzo M, Carbone A, Pastore AL, et al (2016) Experience with allium stent (Triangular Prostatic Stent) for treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia. Neurourol Urodyn 35:S21–S22
Amparore D, De Cillis S, Volpi G et al (2019) First- and second-generation temporary implantable nitinol devices as minimally invasive treatments for bph-related LUTS: systematic review of the literature. Curr Urol Rep 20:47. https://doi.org/10.1007/s11934-019-0912-6
(2020) Temporary Implantable Nitinol Device (iTIND) Demonstrates Reduction In Symptoms and Improved Qol For Men Suffering From Lower Urinary Tract Symptoms Secondary to Benign Prostatic Obstruction - Editorial. https://www.urotoday.com/journal/prostate-cancer-and-prostatic-diseases/from-the-editor/126271-development-of-a-temporary-implantable-nitinol-device-for-patients-with-symptomatic-benign-prostatic-hyperplasia-editorial.html. Accessed 28 Dec 2022
Elterman D, Aubé-Peterkin M, Evans H et al (2022) UPDATE—Canadian Urological Association guideline: male lower urinary tract symptoms/benign prostatic hyperplasia. Can Urol Assoc J 16:245–256. https://doi.org/10.5489/cuaj.7906
Chughtai B, Elterman D, Shore N et al (2021) The iTind temporarily implanted nitinol device for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a multicenter, randomized, controlled trial. J Urol 153:270–276. https://doi.org/10.1097/JU.0000000000002007.10
Amparore D, Fiori C, Valerio M et al (2021) 3-Year results following treatment with the second generation of the temporary implantable nitinol device in men with LUTS secondary to benign prostatic obstruction. Prostate Cancer Prostatic Dis 24:349–357. https://doi.org/10.1038/s41391-020-00281-5
Chughtai B, Kaminetsky J, Shore N et al (2021) Mp01-06 preservation of ejaculatory and erectile function with itind system for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 206:e2–e3. https://doi.org/10.1097/JU.0000000000001962.06
De Nunzio C, Cantiello F, Fiori C et al (2021) Urinary and sexual function after treatment with temporary implantable nitinol device (iTind) in men with LUTS: 6-month interim results of the MT-06-study. World J Urol 39:2037–2042. https://doi.org/10.1007/s00345-020-03418-2
Porpiglia F, Fiori C, Bertolo R et al (2018) 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int 122:106–112. https://doi.org/10.1111/bju.14141
Porpiglia F, Fiori C, Amparore D et al (2019) Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU Int 123:1061–1069. https://doi.org/10.1111/bju.14608
Amparore D, De Cillis S, Fiori C et al (2022) Long term follow-up of an international multicenter prospective study in application of temporary implantable nitinol device (ITIND) in men with lower urinary tract symptoms for BPH. J Urol 207:e1037–e1038. https://doi.org/10.1097/JU.0000000000002669.06
Center for Devices, Radiological Health The Spanner Temporary Prostatic Stent—P060010/S013. In: U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/recently-approved-devices/spanner-temporary-prostatic-stent-p060010s013. Accessed 18 Mar 2023
Corica AP, Larson BT, Sagaz A et al (2004) A novel temporary prostatic stent for the relief of prostatic urethral obstruction. BJU Int 93:346–348. https://doi.org/10.1111/j.1464-410x.2003.04613.x
Shore ND, Dineen MK, Saslawsky MJ et al (2007) A temporary intraurethral prostatic stent relieves prostatic obstruction following transurethral microwave thermotherapy. J Urol 177:1040–1046. https://doi.org/10.1016/j.juro.2006.10.059
Cambio AJ, Roach RM, Arnold P et al (2022) Extended use of the spanner® temporary prostatic stent in catheter-dependent men with comorbidities. Adv Urol 2022:7367851. https://doi.org/10.1155/2022/7367851
Hart J (2021) Zenflow Spring System shows promising results in early trials for patients with BPH. In: Urology Times. https://www.urologytimes.com/view/zenflow-spring-system-shows-promising-results-in-early-trials-for-patients-with-bph. Accessed 27 Dec 2022
The Zenflow Spring System Safety and Performance Study (ZEST CAN). https://clinicaltrials.gov/ct2/show/NCT04309695. Accessed 19 Dec 2022
Woo HH, Huang C-P, Huang WJ et al (2022) The EXPANDER-1 trial: introduction of the novel Urocross™ Expander System for treatment of lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). Prostate Cancer Prostatic Dis 25:576–582. https://doi.org/10.1038/s41391-022-00548-z
Leetun L (2022) New Hope for enlarged prostate: avant enrolls world’s first patient in the ProVIDE clinical trial. https://avanturol.com/new-hope-for-enlarged-prostate-avant-enrolls-worlds-first-patient-in-the-provide-clinical-trial/. Accessed 24 Dec 2022
ProVee Urethral Expander System IDE Study (ProVIDE)—Full Text View—ClinicalTrials.Gov. https://clinicaltrials.gov/ct2/show/NCT05186740?term=ProVIDE+provee&draw=2&rank=1. Accessed 20 Dec 2022
milla1cf (2022) Butterfly Medical announces first BPH clinical study procedures performed in North America for its next generation minimally invasive solution. In: DAIC. https://www.dicardiology.com/content/butterfly-medical-announces-first-bph-clinical-study-procedures-performed-north-america-its. Accessed 27 Dec 2022
Katz R, Ahmed MA, Safadi A et al (2022) MP01-02 the “Butterfly” transurethral retraction device for BPH—over 1 year follow up. J Urol. https://doi.org/10.1097/JU.0000000000002513.02
Katz R, Safadi A, Ahmed MA et al (2021) PD18-11 the “Butterfly”, a novel minimally invasive transurethral retraction device for BPH. J Urol. https://doi.org/10.1097/JU.0000000000002007.11
Charbonnel C, Neuville P, Paparel P et al (2022) Feasibility of EXIME® temporary prosthesis placement and removal in men with acute or chronic urinary retention after failure or inability to selfcatheterize. Prog Urol 32:717–725. https://doi.org/10.1016/j.purol.2022.04.012
Amara N, Al Youssef T, Massa J et al (2022) MP01-01 EXIME® Urethral Stent Implantation (EUSI) as initial treatment for patients presenting with acute urinary retention (AUR) for the first time as a result of BPH. J Urol. https://doi.org/10.1097/JU.0000000000002513.01
Cindolo L, Ferrari R, Rabito S et al (2021) Rezum procedure with Exime® stent: a step forward to micro-invasiveness. Minerva Urol Nephrol 73:273–275. https://doi.org/10.23736/S2724-6051.21.04316-2
Balakrishnan D, Jones P, Somani BK (2020) iTIND: the second-generation temporary implantable nitinol device for minimally invasive treatment of benign prostatic hyperplasia. Ther Adv Urol 12:1756287220934355. https://doi.org/10.1177/1756287220934355
McKenzie P, Badlani G (2011) Critical appraisal of the Spanner TM prostatic stent in the treatment of prostatic obstruction. Med Devices 4:27–33. https://doi.org/10.2147/MDER.S7107
Funding
None.
Author information
Authors and Affiliations
Contributions
AVN contributed to project development, data collection, data analysis, and manuscript writing. IV contributed to data collection, manuscript writing, and manuscript editing. RF contributed to data collection, manuscript writing, and manuscript editing. DDN contributed to project development and manuscript editing. DSE contributed to project development, manuscript editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Elterman is a consultant for Boston Scientific, PROCEPT Biorobotics, Olympus, Urotronic and Prodeon. All other authors do not have any relevant conflicts of interest.
Ethics approval
Ethics approval was not required for this type of study (review).
Informed consent
Formal consent is not required for this type of study (review).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, AL.V., Verma, I., Ferreira, R. et al. A scoping review of office-based prostatic stents: past, present, and future of true minimally invasive treatment of benign prostatic hyperplasia. World J Urol 41, 2925–2932 (2023). https://doi.org/10.1007/s00345-023-04508-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04508-7