Abstract
Purpose
To explore the prognostic importance of metastatic volume in a contemporary daily practice cohort of patients with newly diagnosed metastatic hormone-naive prostate cancer (mHNPC) and to develop a pragmatic prognostic model to predict survival for these patients.
Methods
Since 2014, 113 patients with newly diagnosed mHNPC were prospectively registered. Statistical analysis was performed using SPSS 25.0™ with two-sided p value < 0.05 indicating statistical significance. Univariate and multivariate cox regression analyses were performed to identify prognostic risk factors. Kaplan–Meier method with log-rank statistics was constructed to analyze difference in survival in the prognostic groups. Model performance was assessed using the Concordance-index (C-index) and cross-validated in R v3.4.1. High-volume mHNPC (HVD) was defined as the presence of visceral metastasis or ≥ 4 bone metastases with ≥ 1 appendicular lesion.
Results
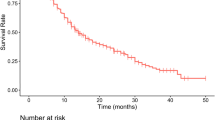
Multivariate analysis identified HVD (p = 0.047) and elevated alkaline phosphatase (ALP) (p = 0.018) as independent prognostic risk factors for overall survival (OS). Consequently, three prognostic groups were created: a good (no risk factors), intermediate (1 risk factor) and poor prognosis group (2 risk factors). Median OS for the good, intermediate and poor prognosis group was not reached, 73 and 20 months (95% CI 9–31 months with p < 0.001 and Correspondence-index of 0.78), respectively.
Conclusions
We developed a pragmatic and qualitative prognostic model consisting of three prognostic risk groups for OS in a daily practice cohort of patients with newly diagnosed mHNPC. Independent prognostic risk factors included in the model were HVD and abnormal ALP.

Similar content being viewed by others
References
Huggins C (1941) Studies on prostatic cancer. Arch Surg 43(2):209. https://doi.org/10.1001/archsurg.1941.01210140043004
Cornford P, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71(4):630–642. https://doi.org/10.1016/j.eururo.2016.08.002
James ND, Spears MR, Clarke NW et al (2015) Survival with newly diagnosed metastatic prostate cancer in the docetaxel era: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 67(6):1028–1038. https://doi.org/10.1016/j.eururo.2014.09.032
Sweeney CJ, Chen Y-H, Carducci M et al (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373(8):737–746. https://doi.org/10.1056/NEJMoa1503747
James ND, Sydes MR, Clarke NW et al (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387(10024):1163–1177. https://doi.org/10.1016/S0140-6736(15)01037-5
Fizazi K, Tran N, Fein L et al (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. https://doi.org/10.1056/nejmoa1704174
James ND, de Bono JS, Spears MR et al (2017) Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. https://doi.org/10.1056/nejmoa1702900
Halabi S, Lin C-Y, Kelly WK et al (2014) Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 32(7):671–677. https://doi.org/10.1200/JCO.2013.52.3696
Van Praet C, Rottey S, Van Hende F et al (2017) Which factors predict overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate post-docetaxel? Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2017.01.019
Smaletz O, Scher HI, Small EJ et al (2002) Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol 20(19):3972–3982. https://doi.org/10.1200/JCO.2002.11.021
Armstrong AJ, Garrett-Mayer ES, Yang Y-CO, de Wit R, Tannock IF, Eisenberger M (2007) A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 13(21):6396–6403. https://doi.org/10.1158/1078-0432.CCR-07-1036
Glass TR, Tangen CM, Crawford ED, Thompson I (2003) Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol 169(1):164–169. https://doi.org/10.1097/01.ju.0000042482.18153.30
Gravis G, Boher J-M, Fizazi K et al (2015) Prognostic factors for survival in noncastrate metastatic prostate cancer: validation of the glass model and development of a novel simplified prognostic model. Eur Urol 68(2):196–204. https://doi.org/10.1016/j.eururo.2014.09.022
Tait C, Moore D, Hodgson C et al (2014) Quantification of skeletal metastases in castrate-resistant prostate cancer predicts progression-free and overall survival. BJU Int 114(6b):E70–E73. https://doi.org/10.1111/bju.12717
Klaff R, Varenhorst E, Berglund A et al (2016) Clinical presentation and predictors of survival related to extent of bone metastasis in 900 prostate cancer patients. Scand J Urol 50(5):352–359. https://doi.org/10.1080/21681805.2016.1209689
Epstein JI, Egevad L, Amin MB et al (2015) The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol 40(2):1. https://doi.org/10.1097/pas.0000000000000530
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71(4):618–629. https://doi.org/10.1016/j.eururo.2016.08.003
Efron B, Tibshirani R (1997) Improvements on cross-validation: the. 632 + Bootstrap method. J Am Stat Assoc 92(438):548–560
Buelens S, Poelaert F, Dhondt B et al (2018) Metastatic burden in newly diagnosed hormone-naive metastatic prostate cancer: comparing definitions of CHAARTED and LATITUDE trial. Urol Oncol Semin Orig Investig 36(4):158.e13–158.e20. https://doi.org/10.1016/j.urolonc.2017.12.009
Funding
This work was supported by the Clinical Research Fund from the Ghent University Hospital.
Author information
Authors and Affiliations
Contributions
SB: data collection/management, data analysis, manuscript writing; EDB, KD, PO, VF and SR: data collection/management, manuscript editing; BD: data collection/management; WV: data analysis, manuscript editing; KDM and CS: manuscript editing; NL: protocol/project development, data collection/management, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the local ethical committee of Ghent (Belgian registration number B670201420709). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buelens, S., De Bleser, E., Dhondt, B. et al. Importance of metastatic volume in prognostic models to predict survival in newly diagnosed metastatic prostate cancer. World J Urol 37, 2565–2571 (2019). https://doi.org/10.1007/s00345-018-2449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2449-6