Abstract
Introduction
Bladder cancer is a major public health concern and the treatment options available are unable to significantly prevent disease recurrence and progression. The need for experimental tumor models to efficiently reproduce the pathology of human cancers has prompted researchers to attempt various approaches.
Methods
A PubMed search combining the MeSH bladder cancer and models was executed in March 2017.
Results
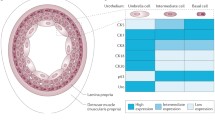
We review the advantages and limitations of currently available in vitro 2D and 3D bladder cancer models as well as in vivo rodent models. To date, despite the description of a variety of animal models (including transplantable, carcinogen-induced and genetically engineered models), the establishment of reliable, simple, practicable and reproducible animal models remains an ongoing challenge. Recently, sophisticated 3D culture systems have been designed to better recapitulate the phenotypic and cellular heterogeneity as well as microenvironmental aspects of in vivo tumor growth, while being more flexible to conduct repeated experiments.
Conclusion
Selecting the most appropriate model for a specific application will maximize the conversion of potential therapies from the laboratory to clinical practice and requires an understanding of the various models available.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30. https://doi.org/10.3322/caac.21332
van der Heijden AG, Witjes JA (2009) Recurrence, progression, and follow-up in non-muscle-invasive bladder cancer. Eur Urol Suppl 8:556–562. https://doi.org/10.1016/j.eursup.2009.06.010
Witjes JA (2006) Management of BCG failures in superficial bladder cancer: a review. Eur Urol 49:790–797. https://doi.org/10.1016/j.eururo.2006.01.017
Crallan RA, Georgopoulos NT, Southgate J (2006) Experimental models of human bladder carcinogenesis. Carcinogenesis 27:374–381. https://doi.org/10.1093/carcin/bgi266
Druckey H (1964) Selective induction of bladder cancer in rats by dibutyl- and N-butyl-N-butanol(4)- nitrosamine. ZeitschriftKrebsforschung 66:280–290
Vasconcelos-Nóbrega C, Colaço A, Lopes C, Oliveira PA (2012) BBN as an urothelial carcinogen. In Vivo 26:727–740
Palmeira C, Oliveira PA, Lameiras C et al (2010) Biological similarities between murine chemical-induced and natural human bladder carcinogenesis. Oncol Lett 1:373–377. https://doi.org/10.3892/ol_00000066
Ding J, Xu D, Pan C et al (2014) Current animal models of bladder cancer: awareness of translatability (review). Exp Ther Med 8:1–9. https://doi.org/10.3892/etm.2014.1837
Pan C-X, Zhang H, Tepper CG et al (2015) Development and characterization of bladder cancer patient-derived xenografts for molecularly guided targeted therapy. PLoS ONE 10:e0134346. https://doi.org/10.1371/journal.pone.0134346
Chan ESY, Patel AR, Smith AK et al (2009) Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J Urol 182:2926–2931. https://doi.org/10.1016/j.juro.2009.08.020
Black PC, Shetty A, Brown GA et al (2010) Validating bladder cancer xenograft bioluminescence with magnetic resonance imaging: the significance of hypoxia and necrosis. BJU Int 106:1799–1804. https://doi.org/10.1111/j.1464-410X.2010.09424.x
Cirone P, Andresen CJ, Eswaraka JR et al (2014) Patient-derived xenografts reveal limits to PI3K/mTOR- and MEK-mediated inhibition of bladder cancer. Cancer Chemother Pharmacol 73:525–538. https://doi.org/10.1007/s00280-014-2376-1
Voskoglou-Nomikos T, Pater JL, Seymour L (2003) Clinical predictive value of the in vitro cell line. Human Xenograft Mouse Allograft Preclin Cancer Models 9:4227–4239
Domingos-Pereira S, Cesson V, Chevalier MF et al (2016) Preclinical efficacy and safety of the Ty21a vaccine strain for intravesical immunotherapy of non-muscle-invasive bladder cancer. OncoImmunology 6:e1265720–e1265727. https://doi.org/10.1080/2162402X.2016.1265720
Grippo PJ, Sandgren EP (2000) Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol 157:805–813. https://doi.org/10.1016/S0002-9440(10)64594-4
Kompier LC, Lurkin I, van der Aa MNM et al (2010) FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE 5:e13821. https://doi.org/10.1371/journal.pone.0013821
Zhang Z-T, Pak J, Wu X-R et al (2001) Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene 20:1973–1980
Zhang Z-T, Pak J, Shapiro E et al (1999) Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma. Can Res 59:3512–3517
Cheng J, Huang H, Zhang Z-T et al (2002) Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Can Res 62:4157–4163
Ahmad I, Singh LB, Foth M et al (2011) K-Ras and B-catenin mutations cooperate with Fgfr3 mutations in mice to promote tumorigenesis in the skin and lung, but not in the bladder. Dis Models Mech 4:548–555. https://doi.org/10.1242/dmm.006874
Zhou H, Liu Y, He F et al (2010) Temporally and spatially controllable gene expression and knockout in mouse urothelium. Am J Physiol Renal Physiol 299:F387–F395. https://doi.org/10.1152/ajprenal.00185.2010
la Pena de FA, Kanasaki K, Kanasaki M et al (2011) Loss of p53 and acquisition of angiogenic MicroRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinoma. J Biol Chem 286:20778–20787
Antoni S, Ferlay J, Soerjomataram I et al (2016) Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. https://doi.org/10.1016/j.eururo.2016.06.010
Sanli O, Dobruch J, Knowles MA et al (2017) Bladder cancer. Nat Publ Group 3:1–19. https://doi.org/10.1038/nrdp.2017.22
Luo KW, Lung WY, Xie C et al (2018) EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3 K/AKT pathway. Oncotarget 9:12261–12272
Zhang X, Chen Y, Dong L, Shi B (2018) Effect of selective inhibition of aquaporin 1 on chemotherapy sensitivity of J82 human bladder cancer cells. Oncol Lett 15:3864–3869. https://doi.org/10.3892/ol.2018.7727
Hennessey PT, Ochs MF, Mydlarz WW et al (2011) Promoter methylation in head and neck squamous cell carcinoma cell lines is significantly different than methylation in primary tumors and xenografts. PLoS One 6:e20584–e20587. https://doi.org/10.1371/journal.pone.0020584
Hutchinson L, Kirk R (2011) High drug attrition rates—where are we going wrong? Nat Publ Group 8:189–190. https://doi.org/10.1038/nrclinonc.2011.34
Sutherland RM (1988) Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177–184
Grimes DR, Kelly C, Bloch K, Partridge M (2013) A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface 11:20131124. https://doi.org/10.1098/rsif.2013.1124
Amaral RLF, Miranda M, Marcato PD, Swiech K (2017) Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol 8:870–875. https://doi.org/10.3389/fphys.2017.00605
Vinci M, Gowan S, Boxall F et al (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 10:29. https://doi.org/10.1186/1741-7007-10-29
Knuchel R, Hofstädter F, Jenkins WEA, Masters JRW (1989) Sensitivities of monolayers and spheroids of the human bladder cancer cell line mgh-u1 to the drugs used for intravesical chemotherapy. Can Res 49:1397–1401
Burgués JP, Gómez L, Pontones JL et al (2007) A chemosensitivity test for superficial bladder cancer based on three-dimensional culture of tumour spheroids. Eur Urol 51:962–970. https://doi.org/10.1016/j.eururo.2006.10.034
Chitcholtan K, Asselin É, Parent S et al (2013) Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res 319:75–87. https://doi.org/10.1016/j.yexcr.2012.09.012
Aljitawi OS, Li D, Xiao Y et al (2013) A novel three-dimensional stromal-based model for in vitro chemotherapy sensitivity testing of leukemia cells. Leuk Lymp 55:378–391. https://doi.org/10.3109/10428194.2013.793323
Gong X, Lin C, Cheng J et al (2015) Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS One 10:e0130348. https://doi.org/10.1371/journal.pone.0130348
Kilani RT, Tamimi Y, Karmali S et al (2002) Selective cytotoxicity of gemcitabine in bladder cancer cell lines. Anticancer Drugs 13:557–566
Bhome R, Bullock MD, Saihati Al HA et al (2015) A top-down view of the tumor microenvironment: structure, cells and signaling. Front Cell Dev Biol 3:1–9. https://doi.org/10.3389/fcell.2015.00033
Wang L-S, Boulaire J, Chan PPY et al (2010) The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials 31:8608–8616. https://doi.org/10.1016/j.biomaterials.2010.07.075
Drury JL, Mooney DJ (2003) Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24:4337–4351. https://doi.org/10.1016/S0142-9612(03)00340-5
Kleinman HK, McGarvey ML, Liotta LA et al (1982) Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21:6188–6193. https://doi.org/10.1021/bi00267a025
Dozmorov MG, Kyker KD, Saban R et al (2006) Analysis of the interaction of extracellular matrix and phenotype of bladder cancer cells. BMC Cancer 6:10–12. https://doi.org/10.1186/1471-2407-6-12
Hauser PJ, Han Z, Sindhwani P, Hurst RE (2007) Sensitivity of bladder cancer cells to curcumin and its derivatives depends on the extracellular matrix. Anticancer Res 27(2):737–740
Thoma CR, Zimmermann M, Agarkova I et al (2014) 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 69–70:29–41. https://doi.org/10.1016/j.addr.2014.03.001
Boxberger H-J, Meyer TF (1994) A new method for the 3-D in vitro growth of human RTll2 bladder carcinoma cells using the alginate culture technique. Biol Cell 82:109–119
Zhang S, Gelain F, Zhao X (2005) Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol 15:413–420. https://doi.org/10.1016/j.semcancer.2005.05.007
El-Sherbiny IM, Yacoub MH (2013) Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract 2013:38. https://doi.org/10.5339/gcsp.2013.38
Baker SC, Shabir S, Southgate J (2014) Biomimetic urothelial tissue models for the in vitro evaluation of barrier physiology and bladder drug efficacy. Mol Pharmaceut 11:1964–1970. https://doi.org/10.1021/mp500065m
Paré B, Touzel-Deschenes L, Lamontagne R et al (2015) Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol Commun 3:1–12. https://doi.org/10.1186/s40478-014-0181-z
Gibot L, Galbraith T, Huot J, Auger FA (2012) Development of a tridimensional microvascularized human skin substitute to study melanoma biology. Clin Exp Metastasis 30:83–90. https://doi.org/10.1007/s10585-012-9511-3
Nyga A, Loizidou M, Emberton M, Cheema U (2013) A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater 9:7917–7926. https://doi.org/10.1016/j.actbio.2013.04.028
Yamato M, Utsumi M, Kushida A et al (2001) Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng Part A 7:1–8
Chabaud S, Bolduc SP (2016) Production of a self-aligned scaffold, free of exogenous material, from dermal fibroblasts using the self-assembly technique. Dermatol Res Pract 2016:1–9. https://doi.org/10.1155/2016/5397319
Ringuette-Goulet C, Bernard G, Chabaud S et al (2017) Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 145:233–241. https://doi.org/10.1016/j.biomaterials.2017.08.041
Nakamura K, Fujiyama C, Tokuda Y et al (2015) Bladder cancer cell implantation in reconstructed bladder in vitro: a model of tumour recurrence. BJU Int 89:119–125
Gibot L, Galbraith T, Bourland J et al (2017) Tissue-engineered 3D human lymphatic microvascular network for in vitro studies of lymphangiogenesis. Nat Protoc 12:1077–1088. https://doi.org/10.1038/nprot.2017.025
Villasante A, Sakaguchi K, Kim J et al (2018) Vascularized tissue-engineered model for studying drug resistance in neuroblastoma. Theranostics 7:4099–4117. https://doi.org/10.7150/thno.20730
Zhu W, Holmes B, Glazer RI, Zhang LG (2015) 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomed Nanotechnol Biol Med. https://doi.org/10.1016/j.nano.2015.09.010
Jeong S-Y, Lee J-H, Shin Y et al (2016) Co-culture of tumor spheroids and fibroblasts in a collagen matrix-incorporated microfluidic chip mimics reciprocal activation in solid tumor microenvironment. PLoS One 11:e0159013–e0159017. https://doi.org/10.1371/journal.pone.0159013
Acknowledgements
We thank Stéphane Chabaud for carefully reading the manuscript. This work was supported by Bladder Cancer Canada Grant to FP and CRG and Ferring Grant to SB. CRG is the recipient of an FRQS Doctoral Research award. FP is the recipient of FRQS Scholarship. SB is the recipient of Canadian Urological Association Scholarship Funds and Canadian Institute for Health Research Grant #258229.
Author information
Authors and Affiliations
Contributions
CRG completed the preparation of table and wrote the manuscript that has been corrected and revised by SB and FP.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Ringuette-Goulet, C., Bolduc, S. & Pouliot, F. Modeling human bladder cancer. World J Urol 36, 1759–1766 (2018). https://doi.org/10.1007/s00345-018-2369-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2369-5