Abstract
Purpose
Over the past 3 decades, no major treatment breakthrough has been reported for advanced bladder cancer. Recent Food and Drug Administration (FDA) approval of five immune checkpoint inhibitors in the management of advanced bladder cancer represent new therapeutic opportunities. This review examines the available data of the clinical trials leading to the approval of ICIs in the management of metastatic bladder cancer and the ongoing trials in advanced and localized settings.
Methods
A literature search was performed on PubMed and ClinicalTrials.gov combining the MeSH terms: ‘urothelial carcinoma’ OR ‘bladder cancer’, and ‘immunotherapy’ OR ‘CTLA-4’ OR ‘PD-1’ OR ‘PD-L1’ OR ‘atezolizumab’ OR ‘nivolumab’ OR ‘ipilimumab’ OR ‘pembrolizumab’ OR ‘avelumab’ OR ‘durvalumab’ OR ‘tremelimumab’. Prospectives studies evaluating anti-PD(L)1 and anti-CTLA-4 monoclonal antibodies were included.
Results
Evidence-data related to early phase and phase III trials evaluating the 5 ICIs in the advanced urothelial carcinoma are detailed in this review. Anti-tumour activity of the 5 ICIs supporting the FDA approval in the second-line setting are reported. The activity of PD(L)1 inhibitors in the first-line setting in cisplatin-ineligible patients are also presented. Ongoing trials in earlier disease-states including non-muscle-invasive and muscle-invasive bladder cancer are discussed.
Conclusions
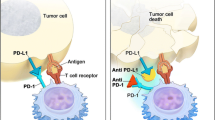
Blocking the PD-1 negative immune receptor or its ligand, PD-L1, results in unprecedented rates of anti-tumour activity in patients with metastatic urothelial cancer. However, a large majority of patients do not respond to anti-PD(L)1 drugs monotherapy. Investigations exploring the potential value of predictive biomarkers, optimal combination and sequences are ongoing to improve such treatment strategies.


Similar content being viewed by others
References
Sternberg CN, de Mulder PH, Schornagel JH, Théodore C, Fossa SD, van Oosterom AT et al (2001) Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 15(19):2638–2646
Witjes AJ, Lebret T, Compérat EM, Cowan NC, De Santis M, Bruins HM et al (2017) Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 71:462–475
Rouprêt M, Neuzillet Y, Masson-Lecomte A, Colin P, Compérat E, Dubosq F et al (2016) CCAFU French national guidelines 2016–2018 on bladder cancer. Prog Urol 27(Suppl 1):S67–S91
Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A (2014) Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(3):40–48
Dash A, Galsky MD, Vickers AJ, Serio AM, Koppie TM, Dalbagni G, Bochner BH (2006) Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107:506–513
Bellmunt J, Théodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G (2009) Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 20:4454–4461
Ortmann CA, Mazhar D (2013) Second-line systemic therapy for metastatic urothelial carcinoma of the bladder. Future Oncol 9:1637–1651
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 25(331):1565–1570
Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14:717–734
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 18(541):321–330
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 9(168):707–723
Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22:333–340
Rouanne M, Loriot Y, Lebret T, Soria JC (2016) Novel therapeutic targets in advanced urothelial carcinoma. Crit Rev Oncol Hematol 98:106–115
Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U et al (2018) Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391(10122):748–757. https://doi.org/10.1016/S0140-6736(17)33297-X
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A et al (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387:1909–1920
Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ et al (2017) Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 3:e172411
Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R et al (2018) Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19:51–64
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J et al (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18:312–322
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L et al (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015–1026
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J et al (2017) Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389:67–76
Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T et al (2017) First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 18:1483–1492
Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J et al (2018) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(Suppl 4):119–142
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S et al (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920–1928
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R (2017) Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 23:4242–4250
Wezel F, Vallo S, Roghmann F (2017) Do we have biomarkers to predict response to neoadjuvant and adjuvant chemotherapy and immunotherapy in bladder cancer? Transl Androl Urol 6:1067–1080
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C et al (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD et al (2017) Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171:540–556
Obara W, Eto M, Mimata H, Kohri K, Mitsuhata N, Miura I et al (2017) A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder. Ann Oncol 28:798–803
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J et al (2010) Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 16:2861–2871
Gough MJ, Crittenden MR (2009) Combination approaches to immunotherapy: the radiotherapy example. Immunotherapy 6:1025–1037
Formenti SC, Demaria S (2013) Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 4:256–265
Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S et al (2014) Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 9:831–838
Acknowledgements
Mathieu Rouanne is the recipient of a Scholarship Funding from the French Urological Association (Association Française d’Urologie)..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest with the contents of this work.
Rights and permissions
About this article
Cite this article
Rouanne, M., Roumiguié, M., Houédé, N. et al. Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. World J Urol 36, 1727–1740 (2018). https://doi.org/10.1007/s00345-018-2332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2332-5