Abstract
Purpose
The purposes of this paper were to present the current status of contrast-enhanced transrectal ultrasound imaging and to discuss the latest achievements and techniques now under preclinical testing.
Objective
Although grayscale transrectal ultrasound is the standard method for prostate imaging, it lacks accuracy in the detection and localization of prostate cancer. With the introduction of contrast-enhanced ultrasound (CEUS), perfusion imaging of the microvascularization became available. By this, cancer-induced neovascularisation can be visualized with the potential to improve ultrasound imaging for prostate cancer detection and localization significantly. For example, several studies have shown that CEUS-guided biopsies have the same or higher PCa detection rate compared with systematic biopsies with less biopsies needed.
Materials and methods
This paper describes the current status of CEUS and discusses novel quantification techniques that can improve the accuracy even further. Furthermore, quantification might decrease the user-dependency, opening the door to use in the routine clinical environment. A new generation of targeted microbubbles is now under pre-clinical testing and showed avidly binding to VEGFR-2, a receptor up-regulated in prostate cancer due to angiogenesis. The first publications regarding a targeted microbubble ready for human use will be discussed.
Conclusion
Ultrasound-assisted drug delivery gives rise to a whole new set of therapeutic options, also for prostate cancer. A major breakthrough in the future can be expected from the clinical use of targeted microbubbles for drug delivery for prostate cancer diagnosis as well as treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most common neoplasm in men. In Europe, in 2008, the incidence rate was 214 cases per 1,000 men, and this number is still rising with, e.g., the increase in age [1]. In the European Union, prostate cancer has about the same incidence as breast cancer [2]. For breast cancer detection, suitable imaging techniques are available and large-scale, image-based, screening programs are active. However, for prostate cancer, no imaging technique currently exists, which can accurately diagnose and stage this malignancy. It is hoped that due to recent developments in imaging, this will change in the near future.
In MRI, functional assessment of the prostate is advancing with techniques such as MR spectrometry, dynamic contrast-enhanced MRI, and diffusion-weighted MRI. Recently, Turkbey et al. [3] showed that with T2-weighted MRI combined with dynamic contrast-enhanced MRI and MR spectrometry at 3T, correct prostate cancer staging could be achieved in 80% of the cases. However, a large difference in, e.g., detection rate is seen between studies depending on patient characteristics and tumor localization [4].
When compared with other medical imaging techniques, ultrasound has many advantages such as the lack of using ionizing radiation. Furthermore, it is cost-effective [5] and can be used at the bedside. However, downsides of ultrasound are, for example, the user dependency and learning curve. Although 3D imaging has already been introduced a long time ago, mostly prostate ultrasound is still performed in 2D.
The goals of this paper were to present the current status of contrast-enhanced transrectal ultrasound imaging and to discuss the latest achievements and techniques now under preclinical testing.
Transrectal ultrasound (TRUS)
TRUS is the classical ultrasound technique for prostate imaging and enables a detailed visualization of the prostate. However, prostate cancer is hard to detect with standard grayscale TRUS. The classical signs for malignancy are hypoechoic lesions, vesicular asymmetry, and capsular irregularity; however, only 11–35% of the malignancies are visible on grayscale TRUS [6]. Furthermore, of all hypoechoic lesions seen, only in 17–57% of the cases malignancy is present [7]. Because of this low accuracy for the diagnosis of PCa, TRUS is mainly used for volume measurement and guidance of systematic biopsies. In conclusion, transrectal grayscale ultrasound imaging lacks accuracy in the diagnosis of prostate cancer, and therefore, there is an urgent need for improvement in TRUS.
In an attempt to increase the diagnostic value of grayscale TRUS, Beerlage et al. tested a system for computerized analysis of ultrasonographic prostate images: AUDEX (Automated Urologic Diagnostic EXpert system). They compared grayscale TRUS images octants with radical prostatectomy specimens. The AUDEX analysis showed a diagnostic accuracy of 57%, and the authors concluded that the system is thus inappropriate for routine clinical use [8].
With the use of computerized transrectal ultrasound (C-TRUS), Loch studied the detection rate of C-TRUS-guided biopsies in men with previous negative systematic biopsies. C-TRUS analyzes static TRUS images by using algorithms based on reflected raw ultrasound data independent of visual grayscale information. In 132 patients, 50% of cancer was detected by C-TRUS-guided biopsies [9]. This is a considerable high value as compared to known numbers of repeated systematic biopsies [10].
Another quantification method developed for PCa detection by TRUS is referred to as Histoscanning. Like C-TRUS, it is a computerized analysis of TRUS data. Braeckman et al. [11] studied 13 men before radical prostatectomy with Histoscanning and showed that in all men, all lesions larger than 0.5 ml were detected. Although promising, more data is needed from different patient groups and different centers to determine the value in the routine clinical environment.
Prostate malignant lesions are thought to consist of stiffer tissue as compared to benign areas. Elastography is a real-time imaging technique that detects these differences in stiffness. Aigner et al. compared elastography-guided biopsies with 10 core systematic biopsies in 94 men. They showed that the detection rate of PCa was comparable, 21.3% in the elastography-guided and 19.1% in the systematic biopsies. With elastography, significantly less biopsies were taken (158 vs. 752) [12].
Prostate cancer induces neovascularistion, which results in a disturbed perfusion of malignant tissue compared to normal prostate tissue [13, 14]. If these changes in tissue blood flow could be visualized, the accuracy for detecting PCa could potentially increase.
The first discovered ultrasound technique for visualization of blood flow was Doppler, which images the relative velocity of blood flow. Sen et al. compared grayscale US with color Doppler in targeted biopsies in 40 patients. Respectively, they show a difference in sensitivity of 88.2 versus 73.5% and specificity of 66.6 versus 33.3% [15]. The main shortcoming of prostate color Doppler imaging is the inability to visualize low blood flow in small vessels, especially in the microvasculature of tissue.
Power Doppler ultrasonography (PDU) increases the sensitivity for the detection of blood flow as compared to color Doppler [16]. Sakayra et al. and Takahashi et al. compared systematic biopsies with PDU imaging. A sensitivity between 77 and 90% was seen, with an specificity of 75–88% [16, 17]. Although color Doppler and power Doppler are more likely to yield positive findings with directed biopsy, still detection is insufficiently sensitive and specific to obviate systematic biopsy. For better imaging of low blood flow and microvascular changes in PCa, contrast-enhanced TRUS imaging was introduced.
Contrast-enhanced ultrasound
The currently used ultrasound contrast agents consist of a solution of gas-filled microbubbles with a diameter in the order of micrometers, smaller than red blood cells, and stabilized with a shell. The bubbles stay inside the blood pool and travel through the whole body through all blood vessels, including the microvasculature. Clinically, contrast-enhanced ultrasound is now mainly used for perfusion measurement of the heart and for the detection of liver malignancies [18, 19].
The first application of contrast-enhanced ultrasound for the diagnosis of prostate cancer was in combination with Doppler imaging. Using contrast-enhanced ultrasound, additional reflectors are added to the blood pool, thereby increasing the sensitivity for detecting low flow in small vessels.
In 2001, Sedelaar et al. showed that increased microvessel density (MVD) in malignant lesions could be detected by contrast-enhanced power Doppler imaging. They found that CEUS-enhanced areas had a 1.93 times higher MVD as compared to the non-enhanced areas. Only small satellite lesions of 1–2 mm diameter were not detected [13].
Frauscher et al. compared contrast-enhanced color Doppler (CECD)-targeted biopsies (CB) with systematic biopsies (SB). In the CB group, 5 or less biopsies were taken from contrast-enhancing areas, and in the SB group, 10 systematic biopsies were taken. Of 230 patients, PCa was detected in 69 patients (30%), with CB in 56 patients (24.4%), and with SB in 52 patients (22.6%). They concluded that the same detection rate can be obtained with less biopsies when CECD-guided biopsies are used [20]. In 2005, Pelzer et al. performed a combined approach of CB and SB to investigate the impact on PCa detection. They also showed that the detection rate is comparable with less biopsies [21]. However, the papers also showed that from all CB in only 1 out of 3 (Pelzer et al. 32%) to 4 (Fraucher et al. 24%), PCa is found.
The last years, new advanced CEUS techniques became available. These so-called contrast-specific imaging techniques enable to differentiate between the non-linear signals reflected by the microbubbles and the linear signals from the tissue. In this way, a contrast-only image can be presented. Several different methods have been developed, such as harmonic imaging, pulse inversion, CPS, and power modulation. The techniques are capable of detecting one single microbubble and therefore can visualize the blood flow in the microvasculature. Figure 1 shows several images of contrast-specific imaging at different moments after the intravenous injection of contrast.
Power modulation CEUS imaging. Each panel: left contrast-only, right normal gray scale imaging. a 20 s after contrast injection. The contrast-only image shows an almost black picture, demonstrating optimal tissue suppression. b Start inflow of contrast 26 s after injection. c Peak enhancement 34 s after injection. d 107 s after injection
Halpern et al. compared contrast-enhanced harmonic imaging-targeted biopsies with systematic biopsy in 301 patients. CEUS-guided biopsies were 2 times more likely to find cancer compared to systematic biopsy in patients with PCa. However, targeted biopsies missed 20% of cancers, which were detected on systematic biopsy alone. They concluded that although the detection rate of carcinoma is higher with CEUS-guided biopsies, systematic biopsies are still needed [22]. Sano et al. used harmonic imaging to perform 12-core systematic and targeted biopsies in 41 patients. They also showed that significantly more cancers are found with CEUS-targeted biopsies: 36.6 versus 17.7% with systematic biopsies. Furthermore, a comparison was made between radical prostatectomy tumor locations and pre-operatively performed CEUS in 13 patients. In 10 patients, at least one tumor could be identified [23]. Matsumoto et al. compared radical prostatectomy specimens with pre-operatively performed grayscale US and harmonic imaging CEUS. In 50 patients, they were able to identify at least one tumor focus in 40% of the cases by grayscale imaging. When using CEUS alone, at least one tumor focus enhancement was seen in 62% of the patients, and when combined, in 80% of the cases, identification of a tumor focus was possible [24].
In conclusion, the new contrast-specific ultrasound techniques show promising results. However, one of the downsides of CEUS is the subjective interpretation by the investigator. For example, several different enhancement patterns can correlate with the presence of PCa, and the most important events to detect these enhancement patterns take place within seconds. This makes the interpretation of CEUS for prostate cancer difficult outside the centers of excellence. To overcome this problem, a more objective and reliable interpretation by quantification of CEUS is needed.
Quantification
The first attempts for quantification of CEUS in prostate cancer used perfusion-related parameters. Goossen et al. used power Doppler techniques to study the in- and outflow characteristics after a bolus injection of contrast. For this, they analyzed the time–intensity curve as measured by the amount of colored pixels related to time. In 78% of the cases, they were able to identify in which side of the prostate the largest malignancy was located by analyzing the time between injection and maximum peak of contrast enhancement [25].
Recently, Zhu et al. investigated the use of hemodynamic parameters measured using harmonic imaging in 103 patients to detect aggressiveness of PCa. ROIs were drawn at systematic biopsy sites and areas of sonographic abnormalities, e.g. heterogenous contrast flow, abnormal Doppler flow, echotexture or contour deformity. The arrival time (AT), time to peak (TTP), and peak intensity for these ROI curves were calculated. High-grade tumors had a significantly shorter AT and TTP than low-grade tumors [26]. No significant difference was detected between the enhancement of low-grade tumors and non-malignant tissue.
We propose an alternative quantification method based on the diffusion or dispersion of contrast agent in the tissue. On a pixel basis, the spreading of contrast in the tissue is determined using a mathematical model of diffusion. This model is fitted to measured time–intensity curves, and a diffusion-related parameter is extracted. We hypothesize that the intravascular diffusion or dispersion, as described by the extracted diffusion parameter, correlates with the microvascular structure and therefore correlates better with angiogenesis than the traditional perfusion parameters. In a preliminary evaluation in 4 patients scheduled for radical prostatectomy, the correlation between the diffusion parameter and the histology was determined. For an example see Fig. 2. Based on a pixel comparison, the area under the ROC curve was 0.909, which demonstrated to be superior to that of any other measured perfusion-related parameter as proposed in literature until now. This promising result has to be further confirmed in larger studies.
In conclusion, quantification could make an objective and reliable interpretation of CEUS possible with a high accuracy. Further scientific and clinical evidence is still needed to judge the role of these techniques in a routine clinical environment. One of the disadvantages is that most of these quantification techniques use 2D contrast imaging. This implies that for every bolus of contrast, only 1 single 2D plane can be quantified. 3D/4D CEUS could solve this limitation, and furthermore enable analysis of perfusion and diffusion in 3D, which most probably will further improve the accuracy of the techniques.
Molecular imaging
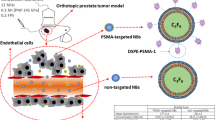
A new generation of ultrasound contrast agent is made of targeted microbubbles. These bubbles have the same general features as traditional microbubbles; however, additional molecules that bind to specific intravascular receptors are embedded in the shell of the bubble. Possible receptor targets for prostate cancer are those that are up-regulated during the process of angiogenesis. Most research has been focusing on the vascular endothelial growth factor (VEGF) receptors.
After an intravenous injection of targeted contrast, bubbles will attach to the target receptors, and after some time, the concentration of free floating microbubbles will be significantly lower than the concentration of the attached bubbles in the tissue where the receptors are up-regulated. After minutes, the attached bubbles can be detected by contrast ultrasound imaging. A great advantage of this technique is the larger time window for detecting lesions; once the bubbles are bound, after approximately 7–10 min, the whole organ can be scanned for minutes.
The last years, investigations using targeted ultrasound contrast have been performed in vitro as well as in vivo [27–30]. Tardy et al. and Fischer et al. used a rat model to investigate the contrast-enhancing effects of target-specific microbubbles versus a non-specific contrast agent in malignant prostatic tissue. They showed that more than 10 min after infusion of a low concentration of VEGFR-2-targeted contrast agent, contrast enhancement is still visible in the prostate. Furthermore, the increase in signal intensity (wash-in rate) and the peak intensity of both the targeted contrast agent and the non-specific contrast agent in malignant tissue was significantly higher than in normal tissue [27, 29]. These experiments in animal models showed the effectiveness and potential use of targeted contrast agents for prostate cancer diagnosis. Unfortunately, most agents use target ligands that cannot be used in humans because of a potentially immunogenic response due to foreign protein content in the shell of the microbubble. However, a recent publication of Pochon et al. investigated a targeted bubble using a biospecific lipopeptide especially designed for use in humans. They demonstrated an avidly bound to cells expressing VEGFR2 in an in vitro human prostate cancer animal model [28]. They concluded that this targeted contrast agent therefore opens the door to clinical use in humans.
In conclusion, pre-clinical research demonstrated the usefulness of targeted bubbles, also in prostate cancer animal models. Recently, reports were published describing target contrast agents designed for clinical use in humans. Therefore, it can be expected that these new targeted agents will become available for clinical testing in short time.
Future
Not only do microbubbles give rise to many new diagnostic imaging possibilities, as described above, but they can also be used to transport certain substances. In ultrasound-assisted drug delivery, microbubbles are filled with particles, e.g. drugs, siRNA, DRA, or stem cells, which then can be released inside the tissue in the ultrasound plane with the help of a high pressure ultrasound burst [31]. This technique is further enhanced by sonoporation, which temporarily increases the cell membrane permeability and, therefore, the drug uptake. Sonoporation describes the process by which the cell permeability is increased by ultrasound in the presence of microbubbles [32].
Discussion
CEUS showed promising results in centers of expertise. One of the most important findings is that with CEUS the number of biopsies can be greatly reduced [20, 21] with a comparable cancer detection rate. Quantification techniques are now developed and introduced that have the potential to increase the accuracy of CEUS analysis and decrease the user dependency. In this way, these techniques can help in making CEUS also available for non-expert centers.
Targeted microbubbles for molecular imaging of prostate cancer have demonstrated promising results in in vitro as well as in vivo animal experiments. An advantage of this technique is that after binding, for several minutes, the whole prostate can be scanned for attached microbubbles, and enough time is available for e.g. targeted biopsies. This implies that with one injection of contrast, the whole prostate can be imaged with 2D contrast-specific imaging. One of the major targets is the VEGFR-2 receptor, which is up-regulated in angiogenesis [27, 29]. It can therefore be hypothesized that more aggressive and faster-growing tumors have an increased VEGFR-2 expression. This would make grading based on imaging possible. Focal therapy and active surveillance are increasingly used. Molecular ultrasound imaging with targeted microbubbles could play a role in selecting patients for the most appropriate treatment. For follow-up, CEUS can also enable visualization of the effects of therapy or medical treatments that influence the perfusion of the prostate (Brachy, radiotherapy, HIFU, cryoablation, or hormonal therapy) and identify early PCa relapses that might not be detected by PSA rise [33].
Conclusion
CEUS has demonstrated excellent results in centers of expertise.
Quantification techniques can improve the accuracy even further and can decrease the user-dependency, opening the door to use in the routine clinical environment.
A major breakthrough in the near future will be the clinical use of targeted microbubbles for prostate cancer diagnosis and treatment.
References
Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N et al (2010) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59:61–71
Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46:765–781
Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, Khurana K et al (2010) Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology 255:89–99
Turkbey B, Albert PS, Kurdziel K, Choyke PL (2009) Imaging localized prostate cancer: current approaches and new developments. AJR Am J Roentgenol 192:1471–1480
Bueschen AJ, Lockhart ME (2011) Evolution of urological imaging. Int J Urol 18:102–112
Beemsterboer PM, Kranse R, de Koning HJ, Habbema JD, Schroder FH (1999) Changing role of 3 screening modalities in the European randomized study of screening for prostate cancer (Rotterdam). Int J Cancer 84:437–441
Engelbrecht MR, Barentsz JO, Jager GJ, van der Graaf M, Heerschap A, Sedelaar JP, Aarnink RGet al (2000) Prostate cancer staging using imaging. BJU Int 86(Suppl 1):123–134
Beerlage HP, Aarnink RG, Ruijter ET, Witjes JA, Wijkstra H, Van De Kaa CA, Debruyne FM et al (2001) Correlation of transrectal ultrasound, computer analysis of transrectal ultrasound and histopathology of radical prostatectomy specimen. Prostate Cancer Prostatic Dis 4:56–62
Loch T (2007) Computerized transrectal ultrasound (C-TRUS) of the prostate: detection of cancer in patients with multiple negative systematic random biopsies. World J Urol 25:375–380
Philip J, Hanchanale V, Foster CS, Javle P (2006) Importance of peripheral biopsies in maximising the detection of early prostate cancer in repeat 12-core biopsy protocols. BJU Int 98:559–562
Braeckman J, Autier P, Soviany C, Nir R, Nir D, Michielsen D, Treurnicht K et al (2008) The accuracy of transrectal ultrasonography supplemented with computer-aided ultrasonography for detecting small prostate cancers. BJU Int 102:1560–1565
Aigner F, Pallwein L, Junker D, Schafer G, Mikuz G, Pedross F, Mitterberger MJ et al (2010) Value of real-time elastography targeted biopsy for prostate cancer detection in men with prostate specific antigen 1.25 ng/ml or greater and 4.00 ng/ml or less. J Urol 184:913–917
Sedelaar JP, van Leenders GJ, Hulsbergen-van de Kaa CA, van der Poel HG, van der Laak JA, Debruyne FM, Wijkstra H et al (2001) Microvessel density: correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol 40:285–293
Fregene TA, Khanuja PS, Noto AC, Gehani SK, Van Egmont EM, Luz DA, Pienta KJ (1993) Tumor-associated angiogenesis in prostate cancer. Anticancer Res 13:2377–2381
Sen J, Choudhary L, Marwah S, Godara R, Marwah N, Sen R (2008) Role of colour Doppler imaging in detecting prostate cancer. Asian J Surg 31:16–19
Takahashi S, Yamada Y, Homma Y, Horie S, Hosaka Y, Kitamura T (2002) Power Doppler ultrasonography-directed prostate biopsy in men with elevated serum PSA levels: an evaluation of the clinical utility and limitations. Urology 60:248–252
Sakarya ME, Arslan H, Unal O, Atilla MK, Aydin S (1998) The role of power Doppler ultrasonography in the diagnosis of prostate cancer: a preliminary study. Br J Urol 82:386–388
Kim TK, Lee KH, Khalili K, Jang HJ (2011) Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging. doi:10.1007/s00261-011-9686-0
Quaia E (2011) Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 21:604–615
Frauscher F, Klauser A, Volgger H, Halpern EJ, Pallwein L, Steiner H, Schuster A et al (2002) Comparison of contrast enhanced color Doppler targeted biopsy with conventional systematic biopsy: impact on prostate cancer detection. J Urol 167:1648–1652
Pelzer A, Bektic J, Berger AP, Pallwein L, Halpern EJ, Horninger W, Bartsch G et al (2005) Prostate cancer detection in men with prostate specific antigen 4–10 ng/ml using a combined approach of contrast enhanced color Doppler targeted and systematic biopsy. J Urol 173:1926–1929
Halpern EJ, Ramey JR, Strup SE, Frauscher F, McCue P, Gomella LG (2005) Detection of prostate carcinoma with contrast-enhanced sonography using intermittent harmonic imaging. Cancer 104:2373–2383
Sano F, Terao H, Kawahara T, Miyoshi Y, Sasaki T, Noguchi K, Kubota Yet al (2010) Contrast-enhanced ultrasonography of the prostate: various imaging findings that indicate prostate cancer. BJU Int. doi:10.1111/j.1464-410X.2010.09735.x
Matsumoto K, Nakagawa K, Hashiguchi A, Kono H, Kikuchi E, Nagata H, Miyajima A et al (2010) Contrast-enhanced ultrasonography of the prostate with Sonazoid. Jpn J Clin Oncol 40:1099–1104
Goossen TE, De La Rosette JJ, Hulsbergen-van de Kaa CA, van Leenders GJ, Wijkstra H (2003) The value of dynamic contrast enhanced power Doppler ultrasound imaging in the localization of prostate cancer. Eur Urol 43:124–131
Zhu Y, Chen Y, Jiang J, Wang R, Zhou Y, Zhang H (2010) Contrast-enhanced harmonic ultrasonography for the assessment of prostate cancer aggressiveness: a preliminary study. Korean J Radiol 11:75–83
Fischer T, Thomas A, Tardy I, Schneider M, Hunigen H, Custodis P, Beyersdorff D et al (2010) Vascular endothelial growth factor receptor 2-specific microbubbles for molecular ultrasound detection of prostate cancer in a rat model. Invest Radiol 45:675–684
Pochon S, Tardy I, Bussat P, Bettinger T, Brochot J, von Schneider WM, Passantino L (2010) BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol 45:89–95
Tardy I, Pochon S, Theraulaz M, Emmel P, Passantino L, Tranquart F, Schneider M (2010) Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Invest Radiol 45:573–578
Xuan JW, Bygrave M, Valiyeva F, Moussa M, Izawa JI, Bauman GS, Klibanov A et al (2009) Molecular targeted enhanced ultrasound imaging of flk1 reveals diagnosis and prognosis potential in a genetically engineered mouse prostate cancer model. Mol Imaging 8:209–220
Schneider M (2008) Molecular imaging and ultrasound-assisted drug delivery. J Endourol 22:795–802
Yoon CS, Park JH (2010) Ultrasound-mediated gene delivery. Expert Opin Drug Deliv 7:321–330
Wondergem N, De La Rosette JJ (2007) HIFU and cryoablation—non or minimal touch techniques for the treatment of prostate cancer. Is there a role for contrast enhanced ultrasound? Minim Invasive Ther Allied Technol 16:22–30
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Smeenge, M., Mischi, M., Laguna Pes, M.P. et al. Novel contrast-enhanced ultrasound imaging in prostate cancer. World J Urol 29, 581–587 (2011). https://doi.org/10.1007/s00345-011-0747-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-011-0747-3