Abstract
Purpose
To evaluate the efficacy and safety of WX-G250, a chimeric monoclonal antibody that binds to carboxy anhydrase IX, combined with low-dose interferon-alpha (LD-IFNα) in patients with progressive metastatic renal cell carcinoma (mRCC).
Patients and methods
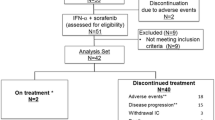
Thirty-one patients, nephrectomized for the primary tumor, clear cell progressive mRCC, were enrolled to receive weekly infusions of WX-G250 (20 mg i.v.; week 2–12) combined with LD-IFNα (3 MIU s.c. 3 times/week; week 1–12). At week 16, patients were evaluated for response and stratified into two groups: (a) responders into the extended treatment group for an additional 6 weeks of treatment or (b) the progressive group with no further study treatment.
Results
Of the 31 treated patients, 26 were evaluable for response to treatment. Two patients showed partial remission and 14 patients had stable disease as assessed in week 16. One patient experienced partial remission resulting in a complete remission lasting at least 17 months. Nine patients had durable stable disease of 24 weeks or longer. Clinical benefit was obtained in 42% (11/26) patients. The median overall survival achieved was 30 months and the 2-year survival was 57%. Patients receiving extended treatment showed a significantly longer 2-year survival rate than discontinued patients (79 vs. 30%; P = 0.0083). In general, treatment was well tolerated with little toxicity.
Conclusion
Treatment with the antibody WX-G250 in combination with LD-IFNα is safe, well tolerated, led to clinically meaningful disease stabilization and demonstrated clinical benefit in this progressive mRCC patient population.

Similar content being viewed by others
References
Siebels M (2007) Epidemiology. In: Siebels M, Stief CG (eds) Neue Therapieansätze beim metastasierten Nierenzellkarzinom, 1st edn. Uni-Med Science, Bremen, pp 18–19
Bleumer I, Oosterwijk E, De Mulder P, Mulders PF (2003) Immunotherapy for renal cell carcinoma. Eur Urol 44:65–75
Figlin RA (1999) Renal cell carcinoma: management of advanced disease. J Urol 161:381–386
Negrier S, Escudier B, Lasset C et al (1998) Recombinant human interleukin-2, recombinant human interferon alpha-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med 338:1272–1278
Stadler WM, Kuzel T, Dumas M, Vogelzang NJ (1998) Multicenter phase II trial of interleukin-2, interferon-alpha, and 13-cis-retinoic acid in patients with metastatic renal-cell carcinoma. J Clin Oncol 16:1820–1825
Atzpodien J, Kirchner H, Illiger HJ et al (2001) IL-2 in combination with IFN- alpha and 5-FU versus tamoxifen in metastatic renal cell carcinoma: long-term results of a controlled randomized clinical trial. Br J Cancer 85:1130–1136
Ravaud A, Delva R, Gomez F et al (2002) Subcutaneous interleukin-2 and interferon alpha in the treatment of patients with metastatic renal cell carcinoma-Less efficacy compared with intravenous interleukin-2 and interferon alpha. Results of a multicenter Phase II trial from the Groupe Francais d’Immunotherapie. Cancer 95:2324–2330
Atzpodien J, Kirchner H, Jonas U et al (2004) Prospectively randomized trial of the german cooperative renal carcinoma chemoimmunotherapy group (DGCIN). Interleukin-2- and interferon alpha-2a based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the german cooperative renal carcinoma chemoimmunotherapy group (DGCIN). J Clin Oncol 22:1188–1194
Oosterwijk E, Ruiter DJ, Hoedemaeker PJ et al (1986) Monoclonal antibody G250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer 38:489–494
Grabmeier K, Vissers JL, De Weijert MC et al (2000) Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer 85:865–870
Surfus JE, Hank JA, Oosterwijk E et al (1996) Anti-renal-cell carcinoma chimeric antibody G250 facilitates antibody-dependent cellular cytotoxicity with in vitro and in vivo interleukin-2-activated effectors. J Immunother Emphasis Tumor Immunol 19:184–191
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma-2. J Clin Oncol 20:289–296
Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal call carcinoma. J Urol 166:1611–1623
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Yang JC, Haworth L, Sherry RM et al (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427–434
Bleumer I, Knuth A, Oosterwijk E et al (2004) A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer 90:985–990
Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC et al (2006) A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol 175:57–62
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for Sunitinib compared with Interferon alpha in patients. J Clin Oncol 27:3584–3590
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, Interferon alpha, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281
Negrier S, Escudier B, Gomez F et al (2002) Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol 13:1460–1468
Acknowledgments
HACA evaluations were performed at Wilex AG, Munich, Germany, which provided the antibody.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research project was sponsored by Wilex AG, Munich, Germany.
Rights and permissions
About this article
Cite this article
Siebels, M., Rohrmann, K., Oberneder, R. et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol 29, 121–126 (2011). https://doi.org/10.1007/s00345-010-0570-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-010-0570-2