Abstract
Background
In acute unilateral renal obstruction, calculated divided renal uptake following injection of tracer may be normal. Divided renal function as measured by uptake may be insensitive to fall in renal plasma flow (RPF) to the obstructed kidney. This study analyses afferent flow rate parameters of optimised models of renogram time activity curves (TAC). Afferent flow rate parameters may have differing sensitivity to altered RPF from divided renal tracer uptake and may be more sensitive to changes in cortical function in renal obstruction.
Method
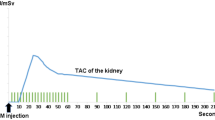
Twenty-four background-corrected renogram TACs using 99mTc-labelled mercapto-acetyl-triglycine (MAG3) with a unilateral obstructive pattern and six normal control renograms TACs were studied. Optimised computed models of each curve were constructed using specialised software (ModelMaker, Cherwell Scientific) and using the Marquardt Least Squares method. Following optimisation to the TAC of each target renogram, the afferent flow rate parameters were calculated.
Results
Following optimisation of models, afferent flow rate parameters, expressed as arbitrary units, (mean 0.15, SD 0.06) in acutely obstructed kidneys, were typically reduced in comparison with those of normal kidneys (mean 0.44, SD 0.04). (Paired t test; P < 0.005). By contrast, this reduction in afferent flow rate parameter was greater than the reduction in differential tracer uptake for the obstructed kidney (divided renal function of the obstructed group; mean 0.3, SD 0.14 compared with the control group; mean 0.45, SD 0.05 (P < 0.05).
Conclusion
Optimised modelling of TACs of obstructed renograms is feasible and may provide a more sensitive index of parenchymal dysfunction in early obstruction than comparing divided renal tracer uptake.



Similar content being viewed by others
References
Gungor F, Anderson P, Gordon I (2002) Effects of the size of regions of interest on the estimation of differential renal function in children with congenital hydronephrosis. Nucl Med Commun 23(2):147–151
Barbalias GA, Nikiforidis G, Vassilakos P, Liatsikos EN (1999) Obstructive uropathy versus nephropathy: compartmental analysis in radioisotopic renography as a new methodology. Urol Res 27:462–469
Dawson P, Peters AM (1994) What is the nephrogram? Br J Radiol 67(793):21–25
Durand E, Blaufox MD, Britton KE, Carlson O, Cosgriff P, Fine E, Fleming J, Nimmon C, Piepsz A, Prigent A, Samal M (2008) International scientific committee of radionuclides in nephrourology (ISCORN) consensus on renal transit time measurements. Semin Nucl Med 38(1):82–102
Peters AM, Gunasekera RD, Henderson BL, Brown J, Lavender JP, de Souza M, Ash JM, Gilday DL (1987) Noninvasive measurement of blood flow and extraction fraction. Nucl Med Commun 8:823–837
Hilson AJW, Maisey MN, Brown CB, Ogg CS, Bewick MS (1978) Dynamic renal transplant imaging with Tc-99 mDTPA (Sn) supplemented by a transplant perfusion index in the management of renal transplants. J Nucl Med 19:994–1000
Coffey JP (2003) Analysis of compartmental models of type 4 nuclear renograms with calculation of flow rate parameters and instantaneous drainage rates. Br J Urology Int 92:85–91
Lupton EW, Lawson RS, Shields RA, Testa HJ (1984) Diuresis renography and parenchymal transit times in the assessment of renal pelvic dilatation. Nucl Med Commun 5(7):451–459
English PJ, Testa HJ, Lawson RS, Carroll RN, Edwards EC (1987) Modified method of diuresis renography for the assessment of equivocal pelviureteric junction obstruction. Br J Urol 59(1):10–14
Cherwell Scientific Ltd (2000) ModelMaker 4 user manual. The Magdalen Centre, Oxford, pp 31–37
Klahr S, Purkerson ML (1994) The pathophysiology of obstructive nephropathy: the role of vasoactive compounds in the hemodynamic and structural abnormalities of the obstructed kidney. Am J Kidney Dis 23(3):219–223
Klahr S, Ishidoya S, Morrisey J (1995) Role of angiotensin II in the tubulointerstitial fibrosis of obstructive nephropathy. Am J Kidney Dis 21(1):141–146
Huang A, Palmer LS, Hom D, Valderrama E, Trachtman H (2000) The role of nitric oxide in obstructive nephropathy. J Urol 163(4):1276–1281
Truong LD, Sheikh-Hamad D, Chakraborty S, Suki WN (1998) Cell apoptosis and proliferation in obstructive uropathy. Sem Nephrol 18(6):641–651
Diamond JR, Richardo SD, Klahr S (1998) Mechanisms of interstitial fibrosis in obstructive nephropathy. Sem Nephrol 18(6):594–602
Aukland K (1980) Methods for measuring renal blood flow: total flow and regional distribution. Ann Rev Physiol 42:265–273
Whitfield HN, Britton KE, Fry IK, Hendry WF, Nimmon CC, Travers P, Wickham JE (1977) The obstructed kidney: correlation between renal function and urodynamic assessment. Br J Urol 49(7):615–619
Whitfield HN, Britton KE, Hendry WF, Nimmon CC, Wickham JE (1978) The distinction between obstructive uropathy and nephropathy by radio-isotope transit times. Br J Urol 50(7):433–436
Gruenwald SM, Nimmon CC, Nawaz MK, Britton KE (1981) A non-invasive gamma-camera technique for the measurement of intrarenal flow distribution in man. Clin Sci 61:385–389
Ham HR (1996) Is renography suitable for deconvolution analysis? J Nucl Med 37:403–404
Kuyvenhoven JD, Ham H, Piepsz A (2001) Is deconvolution applicable to renography? Nucl Med Commun 22:1255–1260
Britton KE, Carroll MJ, Cosgriff PS (2003) Is deconvolution applicable to renography? Nucl Med Commun 24:223–225
Bubeck B, Brandau W, Weber E et al (1990) Pharmacokinetics of technetium 99 m-MAG3 in humans. J Nucl Med 1:1285–1293
Jaffre RA, Britton KE, Nimmon CC, Solanki K, Al-Nahhas A, Bomanji J, Fettich J, Hawkins LA (1988) Technetium 99 m MAG3, a comparison with iodine 123 and iodine 131 orthoiodohippurate in patients with renal disorders. J Nucl Med 29:147–158
Mueller-Suur R, Magnusson G, Bois-Svensson I, Jansson B (1992) Estimation of technetium 99m mercaptoacetyltriglycine plasma clearance by use of one single plasma sample. Eur J Nucl Med 18:28–31
Taylor A Jr, Greene I, Eshima D, Dillehay D (1990) Preliminary results comparing the extraction efficiencies of 131I-OH and 99mTc MAG3 in rat models of ischaemia, renal artery stenosis and cyclosporin toxicity. Contrib Nephrol 79:21–23
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The differential equations describing the model are indicated below, where B is the tracer concentration in the blood compartment, C is tracer concentration in the renal compartment. The afferent and efferent flow constants for the compartments are represented by ka and ke, respectively.
With resolution of these, renal input time function may be obtained as.
Rights and permissions
About this article
Cite this article
Coffey, J.P. Flow rate models in renal obstruction. World J Urol 29, 109–114 (2011). https://doi.org/10.1007/s00345-010-0569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-010-0569-8