Abstract
Introduction
The restoration of erectile function following complete transection of nerve tissue during surgery remains challenging. Recently, graft procedures using sural nerve grafts during radical prostatectomy have had favorable outcomes, and this has rekindled interest in the applications of neural repair in a urologic setting. Although nerve repair using autologous donor graft is the gold standard of treatment currently, donor nerve availability and the associated donor site morbidity remain a problem. In this study, we investigated whether an “off-the-shelf” acellular nerve graft would serve as a viable substitute. We examined the capacity of acellular nerve scaffolds to facilitate the regeneration of cavernous nerve in a rodent model.
Materials and methods
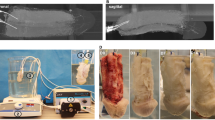
Acellular nerve matrices, processed from donor rat corporal nerves, were interposed across nerve gaps. A total of 80 adult male Sprague-Dawley rats were divided into four groups. A 0.5-cm segment of cavernosal nerve was excised bilaterally in three of the four groups. In the first group, acellular nerve segments were inserted bilaterally at the defect site. The second group underwent autologous genitofemoral nerve grafts at the same site, and the third group had no repair. The fourth group underwent a sham procedure. Serial cavernosal nerve function assessment was performed using electromyography (EMG) at 1 and 3 months following initial surgery. Histological and immunocytochemical analyses were performed to identify the extent of nerve regeneration.
Results
Animals implanted with acellular nerve grafts demonstrated a significant recovery in erectile function when compared with the group that received no repair, both at 1 and 3 months. EMG of the acellular nerve grafts demonstrated adequate intracavernosal pressures by 3 months (87.6% of the normal non-injured nerves). Histologically, the retrieved regenerated nerve grafts demonstrated the presence of host cell infiltration within the nerve sheaths. Immunohistochemically, antibodies specific to axons and Schwann cells demonstrated an increase in nerve regeneration across the grafts over time. No organized nerve regeneration was observed when the cavernous nerve was not repaired.
Conclusion
These findings show that the use of nerve guidance channel systems allow for accelerated and precise cavernosal nerve regeneration. Acellular nerve grafts represent a viable alternative to fresh autologous grafts in a rodent model of erectile dysfunction.




Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2007) Cancer statistics. CA Cancer J Clin 57:43
Miller DC, Gruber SB, Hollenbeck BK et al (2006) Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst 98:1134 See comment
Potosky AL, Davis WW, Hoffman RM et al (2004) Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 96:1358 See comments
Walsh PC, Lepor H, Eggleston JC (1983) Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate 4:473
Walsh PC (1998) Anatomic radical retropubic prostatectomy. In: Walsh PC, Retik AB, Vaughan EDJ (eds) Campbell’s urology, vol 3, 7th edn. Saunders, Philadelphia, pp 2565–2588
Mullerad M, Donohue JF, Li PS et al (2006) Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med 3:77
Kim ED, Scardino PT, Hampel O et al (1999) Interposition of sural nerve restores function of cavernous nerves resected during radical prostatectomy. J Urol 161:188
Kim ED, Scardino PT, Kadmon D et al (2001) Interposition sural nerve grafting during radical retropubic prostatectomy. Urology 57:211
Kim ED, Nath R, Kadmon D et al (2001) Bilateral nerve graft during radical retropubic prostatectomy: 1-year followup. J Urol 165:1950
Kim ED, Nath R, Slawin KM et al (2001) Bilateral nerve grafting during radical retropubic prostatectomy: extended follow-up. Urology 58:983
Evans GR (2001) Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec 263:396
Gulati AK (1988) Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J Neurosurg 68:117
Ide C (1996) Peripheral nerve regeneration. Neurosci Res 25:101
Kim BS, Baez CE, Atala A (2000) Biomaterials for tissue engineering. W J Urol 18:2
Hadlock T, Elisseeff J, Langer R et al (1998) A tissue-engineered conduit for peripheral nerve repair. Arch Otolaryngol Head Neck Surg 124:1081
Hadlock TA, Sundback CA, Hunter DA et al (2001) A new artificial nerve graft containing rolled Schwann cell monolayers. Microsurgery 21:96
Evans GR, Brandt K, Widmer MS et al (1999) In vivo evaluation of poly(l-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials 20:1109
Evans GR, Brandt K, Widmer MS et al (1998) Poly(dl-lactic-co-glycolic) acid (PGLA) biodegradable tissue engineered nerve conduits: their use in peripheral nerve regeneration. Eur Tissue Repair Soc Bull 5:22
Evans GR (2000) Challenges to nerve regeneration. Semin Surg Oncol 19:312
Atala A (2007) Engineering tissues, organs and cells. J Tissue Eng Regen Med 1:83
Chen F, Yoo JJ, Atala A (1999) Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology 54:407
Sondell M, Lundborg G, Kanje M (1998) Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 795:44
Kim BS, Yoo JJ, Atala A (2003) Peripheral nerve regeneration using acellular nerve grafts. J Biomed Res 68:209
Fujioka M, Tasaki I, Kitamura R et al (2007) Cavernous nerve graft reconstruction using an autologous nerve guide to restore potency. BJU Int 100:1107
Namiki S, Saito S, Nakagawa H et al (2007) Impact of unilateral sural nerve graft on recovery of potency and continence following radical prostatectomy: 3-year longitudinal study. J Urol 178:212
Widmer MS, Gupta PK, Lu L et al (1998) Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials 19:1945
Mikos AG, Thorsen AJ, Czerwonka LA et al (1994) Preparation of poly(glycolic) acid bonded fiber structures for cell attachment and transplantation. Polymer 35:1068
Molander H, Olsson Y, Engkvist O et al (1982) Regeneration of peripheral nerve through a polyglactin tube. Muscle Nerve 5:54
Quinlan DM, Nelson RJ, Partin AW et al (1989) The rat as a model for the study of penile erection. J Urol 141:656
Chen KK, Chan JY, Chang LS et al (1992) Intracavernous pressure as an experimental index in a rat model for the evaluation of penile erection. J Urol 147:1124
Chen M-H, Chen P-R, Chen M-H, Hsieh S-T, Lin F-H, Lin F (2006) Gelatin-tricalcium phosphate membranes immobilized with NGF, BDNF, or IGF-1 for peripheral nerve repair: an in vitro and in vivo study. J Biomed Mat Res A 79A:846
Pfister LA, Alther E, Papaloizos M et al (2008) Controlled nerve growth factor release from multi-ply alginate/chitosan-based nerve conduits. Eur J Pharm Biopharm 69:563
Griffin CG, Letourneau PC (1980) Rapid retraction of neurites by sensory neurons in response to increased concentrations of nerve growth factor. J Cell Biol 86:156
Rich KM, Luszczynski JR, Osborne PA et al (1987) Nerve growth factor protects adult sensory neurons from cell death and atrophy caused by nerve injury. J Neurocytol 16:261
Arnett HA, Mason J, Marino M et al (2001) TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4:1116
Daigle JL, Hong JH, Chiang CS et al (2001) The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res 61:8859
Atala A, Bauer SB, Soker S et al (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367:1241
De Filippo RE, Yoo JJ, Atala A (2002) Urethral replacement using cell seeded tubularized collagen matrices. J Urol 168:1789
Halum SL, Naidu M, Delo DM et al (2007) Injection of autologous muscle stem cells (myoblasts) for the treatment of vocal fold paralysis: a pilot study. Laryngoscope 117:917
Kwon TG, Yoo JJ, Atala A (2002) Autologous penile corpora cavernosa replacement using tissue engineering techniques. J Urol 168:1754
Paltiel HJ, Diamond DA, Zurakowski D et al (2004) Endoscopic treatment of vesicoureteral reflux with autologous chondrocytes: postoperative sonographic features. Radiology 232:390
Salgado AJ, Oliveira JT, Pedro AJ et al (2006) Adult stem cells in bone and cartilage tissue engineering. Curr Stem Cell Res Ther 1:345
Gao J, Yao JQ, Caplan AI (2007) Stem cells for tissue engineering of articular cartilage. Proceedings of the Institution of Mechanical Engineers. Part H, J Eng Med 221: 441
Ruszczak Z (2003) Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev 55:1595
Acknowledgments
The authors would like to thank Dr. Karl-Erik Andersson and Dr. Jennifer Olson for editorial assistance.
Conflict of interest statement
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Connolly, S.S., Yoo, J.J., Abouheba, M. et al. Cavernous nerve regeneration using acellular nerve grafts. World J Urol 26, 333–339 (2008). https://doi.org/10.1007/s00345-008-0283-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-008-0283-y