Abstract
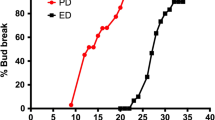
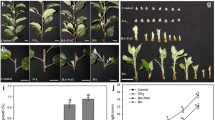
Auxin–cytokinin (CK) interactions have been extensively studied in the control of bud outgrowth in herbaceous plants. However, in temperate woody plants where the meristem of dormant buds can be repressed by either exogenous or endogenous factors, abscisic acid (ABA) has been suggested as a potential regulator of bud outgrowth. To investigate the involvement of ABA, CK, and auxin on bud sprouting in Vitis vinifera, single-bud cuttings were used under forced conditions. This artificial bud sprouting system mimics and hastens the natural sprouting process that occurs in spring. Our results showed that expression of the ABA biosynthesis gene VvNCED1 decreased during incubation, whereas expression of the ABA catabolism gene VvA8H3 remained unaltered. Expression of CK biosynthesis-related genes ISOPENTENYL TRANSFERASE (VvIPTs) and LONELY GUY (VvLOG1), CK catabolism-related gene CYTOKININ OXIDASE (VvCKX3), and key auxin biosynthesis gene VvYUC3 increased with incubation time. Moreover, treatment with hydrogen cyanamide (HC), a compound that breaks vine latency, increased expression of VvIPTs and VvLOG1 and reduced expression of VvCKX3 and VvNCED1. These results are consistent with previous reports indicating that HC increases CK levels and decreases ABA levels in grapevine buds. Taken together, the results suggest that in the vine, bud sprouting is preceded by a decrease in ABA content and an increase in CK and auxin levels.







Similar content being viewed by others
References
Azizi P, Rafi MY, Maziah M, Abdullah SNA, Hanafi MM, Latif MA, Rashid AA, Sahebi M (2015) Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mech Dev 135:1–15
Bennet T, Sieberer T, Willet B, Booker J, Lusching C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16:553–563
Brewer PB, Dun EA, Gui, R, Mason MG, Beveridge C (2015) Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol 168:1820–1829
Brown BT, Foster C, Phillips JN, Rattigan BM (1979) The indirect role of 2,4-D in the maintenance of apical dominance in decapitated sunflower seedlings (Helianthus annuus L.). Planta 146:475–480
Brugière N, Shuping J, Hantke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE (2003) Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, absicisic acid and abiotic stress. Plant Physiol 132:1228–1240
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Crabbe JJ (1984) Correlative effects modifying the course of bud dormancy in woody plants. Z Planzenphysiol 113: 465–469
Crawford S, Shinohara N, Siberee T (2010) Strigolactone enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913
Dennis FG (2003) Problems in standardizing methods for evaluating the chilling requirements for the breaking of dormancy in buds of woody plants. HortScience 38:347–350
Dun EA, de Saint Germain A, Rameau C, Beveridge C (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158:487–498
Fennel A, Hoover E (1991) Photoperiod influences growth, bud dormancy and cold acclimation of Vitis labruscana and V. riparia. J Am Soc Hort Sci 116:270–273
Foo E, Bullier E, Goussot M, Foucher M, Rameau C, Beveridge C (2005) The Branching gene RAMOUSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17:464–474
Gegas VC, Doonan JH (2006) Expression of cell cycle genes in shoot apical meristem. Plant Mol Biol 60: 947–961
Gocal GFW, Pharis RP, Yeung EC, Pearce D (1991) Changes after decapitation in concentration of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv tender green. Plant Physiol 95:344–350
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danous S, Portrais JC, Bowmeester H, Bécaud G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Grant TNL, Gargrave J, Dami IE. 2013. Morphological, Physiological, and Biochemical changes in Vitis genotypes in response to photoperiod regimes. Am J Enol Vitic 64: 466–475
Hall SM, Hillman JR (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. Timing of bud growth following decapitation. Planta 123:137–143
He D, Mathiason K, Fennell (2012) Auxin cytokinin related gene expression during active shoot growth and latent bud paradormancy in Vitis riparia grapevine. J Plant Physiol 169: 643–648
Herber G, Kieber JJ (2002) Cytokinin new insights into a classical phytohormone. Plant Physiol 128:354–362
Hyward A, Stirnberg P, Beveridge C, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412
Koussa T, Broquedis M, Bouard J.1994. Changes of abscisic acid level during the development of grape latent buds, particularly in the phase of dormancy break. Vitis 33:63–67
Kühn N, Ormeño-Nuñez J, Jaque-Zamora G, Pérez FJ (2009) Photoperiod modifies the diurnal expression profile of VvPHYA and VvPHYB transcripts in field-grown grapevine leaves. J Plant Physiol 166:1172–1180
Kurakawa T, Ueda N, MaeKawa M, Kobayashi K, Kojima M, Nagato Y (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445:555–562
Lang GA, Early JD, Martin GC, Darnell RL (1987) Endo-para and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22:381–387
Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion Polla A (2006) Functional analysis of Arabidopsis NCED genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45:309–319
Li CJ, Guevera E, Herrera J, Bengerth F (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94:465–469
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the ∆∆CT method. Methods 25:402–408
Lombard PJ, Cook NC, Bellstedt DU (2006) Endogenous cytokinin levels of table grape vine during spring budburst as influenced by hydrogen cyanamide application and pruning. Sci Hortic 109:92–96
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:3853–3872
McIntyre GI, Damson EL (1988) Apical dominance in Phaseolus vulgaris. The triggering effect of shoot decapitation and leaf excision on growth of the lateral buds. Physiol Plant 74:607–614
Müller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107:1203–1212
Noriega X, Burgos B, Pérez FJ (2007) Short-day photoperiod triggers and low temperature increase expression of peroxidase RNA transcripts and basic peroxidase isoenzyme activity in grape-buds. Phytochemistry 68: 1376–1383
Parada F, Noriega X, Dantas D, Bressan-Smith R, Pérez FJ (2016) Differences in respiration between dormant and non-dormant buds suggest the involvement of ABA in the development of endodormancy in grapevines. J Plant Physiol 201:71–78
Rohde A, Bhalereao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12:217–223
Roman H, Girault T, Barbier F, Péron T, Brouard N, Pencick A, Novák O, Vian A, Sakr S, Lothier J, Le Gourrierec, Leduc N (2016) Cytokinin are initial targets of light in the control of bud outgrowth. Plant Physiol 172:489–509
Rozen S, Skaletsky H (2000) Primer3 on the www for general users and for biologist programmers. Methods Mol Biol 132:365–386
Sachs T (1981) The control of patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Shimizu-Sato S, Mori H (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol 127:1405–1413
Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69:429–435
Sreekantan L, Mathiason L, Grimplet J, Schlauch K, Dickerson JA, Fennell A (2010) Differential floral developmentand gene expression in grapevines during long and short photoperiods suggest a role for floral genes in dormancy transitioning. Plant Mol Biol 73:191–205
Srinivasan C, Mullins MG (1978) Control of flowering in the grapevine (Vitis vinifera L). Plant Physiol 61:127–130
Srinivasan C, Mullins MG (1980) Flowering in Vitis: effects of genotype on cytokinin-induced conversion of tendrils into inflorescence. Vitis 19:293–300
Thimann KV, Skoog F (1934) On the inhibition of bud development and other functions of growth substances in Vicia faba. Proc R Soc Lond B Biol Sci 114:317–339
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magone H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Vergara R, Pérez FJ (2010) Similarities between natural and chemically induced bud endodormancy release in grapevine Vitis vinifera L. Sci Hortic 125:648–653
Wake CMF, Fennell A (2000) Morphological, physiological and dormancy responses of three Vitis genotype to short photoperiod. Physiol Plant 109:203–210
Yoshida S, Mandel T, Kuhlemeier C (2011) Stem cell activation by light guides plant organogenesis. Genes Dev 25:1439–1450
Young PR, Lashbrooke JG, Alexandersson E, Jacobson D, Moser C, Velasco R, Vivier MA (2012) The gene and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genom 13:243–259
Zheng C, Halaly T, Acheampong AK, Takebayashi Y, Jikumaru Y, Kamiya Y, Or E (2015) Absicisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act trough modification of ABA metabolism. J Exp Bot 66:1527–1542
Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L (2006) The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation in the outgrowth of axillary buds. Plant J 48:667–698
Acknowledgements
The financial support of FONDECYT project 1140318 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noriega, X., Pérez, F.J. ABA Biosynthesis Genes are Down-regulated While Auxin and Cytokinin Biosynthesis Genes are Up-regulated During the Release of Grapevine Buds From Endodormancy. J Plant Growth Regul 36, 814–823 (2017). https://doi.org/10.1007/s00344-017-9685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9685-7