Abstract
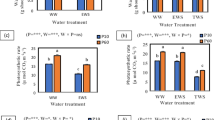
The aim of this work was to investigate the balance between the activities of ascorbate peroxidase (APX) and phenol peroxidases (POD) and cowpea root growth in response to dehydration and salt stress. Root growth and indicators of oxidative response were markedly changed in response to salinity and dehydration. Salt treatment strongly inhibited root elongation, which was associated with an increase in lignin content and a significant decrease in the concentrations of apoplastic hydrogen peroxide (H2O2) and ascorbate. In conditions of extreme salinity, cytosol–APX activity was significantly decreased. In contrast, cell-wall POD activity was greatly increased, whereas lipid peroxidation was unchanged. These results indicate that POD could be involved in both H2O2 scavenging and the inhibition of root elongation under high salinity. In contrast, dehydration stimulated primary root elongation and increased lipid peroxidation and apoplastic ascorbate content, but it did not change APX and POD activities or H2O2 concentration. When cowpea roots were subjected to salinity followed by dehydration, the water and pressure potentials were decreased, and lipid peroxidation was markedly increased, highlighting the additive nature of the inhibitory effects caused by salt and dehydration. The proline concentration was markedly increased by dehydration alone, as well as by salt followed by dehydration, suggesting a possible role for proline in osmotic adjustment. Salinity and dehydration induce contrasting responses in the growth and morphology of cowpea roots. These effects are associated with different types of oxidative modulation involving cytosolic-APX and cell-wall POD activities and apoplast H2O2 and ascorbate levels.








Similar content being viewed by others
References
Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plantarum 85:235–241
Bacon MA, Thompson DS, Davies WJ (1997) Can cell wall peroxidase activity explain the leaf growth response of Lolium temulentum L. during dehydration? J Exp Bot 48:2075–2085
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–209
Bindschedler LV, Cewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davis DR, Ausubel FM, Bolwell GP (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47:851–863
Bonifacio A, Martins MO, Ribeiro CW, Fontenele AV, Carvalho FEL, Margis-Pinheiro M, Silveira JAG (2011) Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ 34:1705–1722
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Cakmak I, Horst WJ (1991) Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571
Cavalcanti FR, Lima JPMS, Ferreira-Silva SL, Viégas RA, Silveira JAG (2007) Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J Plant Physiol 164:591–600
Cosio C, Dunand C (2009) Specific functions of individual class III peroxidase genes. J Exp Bot 60(2):391–408
Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24:275–287
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Angel Torres M, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 6930:442–446
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Kampfenkel K, Van Montagu M, Inze D (1995) Effects of iron excess on Nicotiana plumbaginifolia plants. Implications to oxidative stress. Plant Physiol 107:725–735
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O •−2 , H2O2, and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:1–10
Maia JM, Macêdo CEC, Voigt EL, Freitas JBS, Silveira JAG (2010a) Antioxidative enzymatic protection in leaves of two contrasting cowpea cultivars under salinity. Biol Plant 54:159–163
Maia JM, Macêdo CEC, Voigt EL, Freitas JBS, Ferreira-Silva SL, Silveira JAG (2010b) Salt-induced changes in antioxidative enzyme activities in root tissues do not account for the differential salt tolerance of two cowpea cultivars. Braz J Plant Physiol 22(1):113–122
Maia JM, Ferreira-Silva SL, Voigt EL, Macêdo CEC, Ponte LFA, Silveira JAG (2012) Atividade de enzimas antioxidantes e inibição do crescimento radicular de feijão caupi sob diferentes níveis de salinidade. Acta Bot Bras 26(2):331–338
Mandal S, Das RK, Mishra S (2011) Differential occurrence of oxidative burst and antioxidative mechanism in compatible and incompatible interactions of Solanum lycopersicum and Ralstonia solanacearum. Plant Physiol Biochem 49:117–123
Marjamaa K, Kukkola EM, Fagerstedt KV (2009) The role of xylem class III peroxidases in lignification. J Exp Bot 60:367–376
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during dehydration and salinity stresses. Plant Cell Environ 33:453–467
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Monties B (1989) Lignins. In: Dey PM, Harbone JB (eds) Methods in plant biochemistry. Academic Press, New York, pp 113–158
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Neves GYS, Marchiosi R, Ferrarese MLL, Siqueira-Soares RC, Ferrarese-Filho O (2010) Root growth inhibition and lignification induced by salt stress in soybean. J Agron Crop Sci 196:467–473
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Passardi F, Tognolli M, de Meyer M, Penel C, Dunand C (2006) Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta 223:965–974
Pedreira J, Herrera MT, Zarra I, Revilla G (2011) The overexpression of AtPrx37, an apoplastic peroxidase, reduces growth in Arabidopsis. Physiol Plant 141(2):177–187
Petricka JJ, Winter CM, Benfey PN (2012) Control of arabidopsis root development. Annu Rev Plant Biol 63:563–590
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide: the response of arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Rocha IMA, Vitorello VA, Silva JS, Ferreira-Silva SL, Viégas RA, Silva EN, Silveira JAG (2012) Exogenous ornithine is an effective precursor and the δ-ornithine amino transferase pathway contributes to proline accumulation under high N recycling in salt-stressed cashew leaves. J Plant Physiol 169:41–49
Sánchez M, Queijeiro E, Revilla G, Zarra I (1997) Changes in ascorbate levels in apoplastic fluid during growth of pine hypocotyls. Effect on peroxidase activities associated with cell walls. Physiol Plantarum 101:815–820
Scholander PF, Hammel HT, Bradstreet ED, Hemminbsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Sharp RE, Davies WJ (1989) Regulation of growth and development of plants growing with restrict supply of water. In: Jones HG, Flowers TJ, Jones MB (eds) Plants under stress. Cambridge University Press, Cambridge, pp 71–93
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55(407):2343–2351
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Silva EM, Ferreira-Silva SL, Fontenele AV, Ribeiro RV, Viégas RA, Silveira JAG (2010) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167:1157–1164
Silveira JAG, Araújo SAM, Lima JPMS, Viégas RA (2009) Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in atriplex nummularia. Environ Exp Bot 66:1–8
Silveira JAG, Costa RCL, Viégas RA, Oliveira JTA, Figueiredo VBM (2003) N-compound accumulation and carbohydrate shortage on N2 fixation in drought-stressed and rewatered cowpea plants. Span J Agric Res 1(2):65–75
Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21:197–203
Spollen WG, Sharp RE, Saab IN, Wu Y (1993) Regulation of cell expansion in roots and shoots at low water potentials. In: Smith JAC, Griffiths H (eds) Water deficits. Plant responses from cell to community. Bios Scientific Publishers, Oxford, pp 37–52
Sugiyama T, Goto Y, Akasawa T (1968) Pyruvate kinase activity of wheat plants grown under potassium deficient conditions. Plant Physiol 43:730–734
Takahama U, Oniki T (1994) The association of ascorbate and ascorbate oxidase in the apoplast with IAA-enhanced elongation of epicotyls from Vigna angularis. Plant Cell Physiol 35:257–266
Turk KJ, Hall AE (1980) Drought adaptation of cowpea. IV: Influence of drought on water use and relation with growth and seed yield. Agron J 72:440–448
Warm E, Laties GG (1982) Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminol. Phytochemistry 21:827–831
Wu Y, Cosgrove DJ (2000) Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot 51:1543–1553
Yamaguchi M, Sharp RE (2010) Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant Cell Environ 33:590–603
Zhou B, Guo Z, Liu Z (2006) A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul 49:113–118
Zhu JK (2002) Salt and dehydration stress signal transduction in plants. Ann Rev Plant Biol 53:247–273
Acknowledgments
We acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) for financial support. JAGS is a researcher for CNPq, and JMM, ELV, and AVF were granted CNPq student fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maia, J.M., Voigt, E.L., Ferreira-Silva, S.L. et al. Differences in Cowpea Root Growth Triggered by Salinity and Dehydration are Associated with Oxidative Modulation Involving Types I and III Peroxidases and Apoplastic Ascorbate. J Plant Growth Regul 32, 376–387 (2013). https://doi.org/10.1007/s00344-012-9308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9308-2