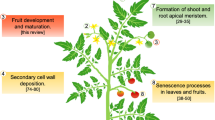
Abstract
The ripening of fleshy fruits represents the unique coordination of developmental and biochemical pathways leading to changes in color, texture, aroma, and nutritional quality of mature seed-bearing plant organs. The gaseous plant hormone ethylene plays a key regulatory role in ripening of many fruits, including some representing important contributors of nutrition and fiber to the diets of humans. Examples include banana, apple, pear, most stone fruits, melons, squash, and tomato. Molecular exploration of the role of ethylene in fruit ripening has led to the affirmation that mechanisms of ethylene perception and response defined in the model system Arabidopsis thaliana are largely conserved in fruit crop species, although sometimes with modifications in gene family size and regulation. Positional cloning of genes defined by ripening defect mutations in the model fruit system tomato have recently led to the identification of both novel components of ethylene signal transduction and unique transcription factor functions influencing ripening-related ethylene production. Here we summarize recent developments in the regulation of fruit ripening with an emphasis on the regulation of ethylene synthesis, perception, and response.


Similar content being viewed by others
References
Adams-Phillips L, Barry C, Giovannoni J. 2004a. Signal transduction systems regulating fruit ripening. Trends Plant Sci 9:331–338
Adams-Phillips L, Barry C, Kannan P, Leclercq J, Bouzayen M, Giovannoni J. 2004b. Evidence that CTR1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Mol Biol 54:387–404
Aharoni A, Keizer LC, van-den-Broeck HC, Blanco-Portales R, Munoz-Blanco J, Bois G, Smit P, De-Vos RC, O’Connell AP. 2002. Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiol 129:1019–1031
Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ. 2005. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17:2954–2965
Alonso JM, Chamarro J, Granell A. 1995. Evidence for the involvement of ethylene in the expression of specific RNAs during maturation of the orange, a nonclimacteric fruit. Plant Mol Biol 29:385–390
Ayub R, Guis M, BenAmor M, Gillot L, Roustan JP, Latche A, Bouzayen M, Pech JC. 1996. Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nat Biotechnol 14:862–866
Barry CS, Giovannoni JJ. 2006. Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc Natl Acad Sci U S A 103:7923–7928
Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. 1996. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J 9:525–535
Barry CS, Llop-Tous MI, Grierson D. 2000. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123:979–986
Barry CS, McQuinn RP, Thompson AJ, Seymour GB, Grierson D, Giovannoni JJ. 2005. Ethylene insensitivity conferred by the Green-ripe and Never-ripe 2 ripening mutants of tomato. Plant Physiol 138:267–275
Bauchot A, Mottram D, Dodson A, John P. 1998. Effect of aminocyclopropane-1-carboxylic acid oxidase antisense gene on the formation of volatile esters in Cantaloupe Charentais melon (cv. Vedrantais). J Agric Food Chem 46:4787–4792
Bleecker AB, Kende H. 2000. Ethylene: A gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Bleecker AB, Estelle MA, Somerville C, Kende H. 1988. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241:1086–1089
Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. 1993. Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 262:539–544
Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker J-R. 1997. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89:1133–1144
Chen G, Alexander L, Grierson D. 2004a. Constitutive expression of EIL-like transcription factor partially restores ripening in the ethylene-insensitive Nr tomato mutant. J Exp Bot 55:1491–1497
Chen GP, Hackett R, Walker D, Taylor A, Lin ZF, Grierson D. 2004b. Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol 136:2641–2651
Chervin C, El-Kereamy A, Roustan JP, Latche A, Lamon J, Bouzayen M. 2004. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci 167:1301–1305
Ciardi JA, Tieman DM, Jones JB, Klee HJ. 2001. Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe Interact 14:487–495
Clark KL, Larsen PB, Wang X, Chang C. 1998. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci U S A 95:5401–5406
Dandekar AM, Teo G, Defilippi BG, Uratsu SL, Passey AJ, Kader AA, Stow JR, Colgan RJ, James DJ. 2004. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res 13:373–384
Defilippi BG, Dandekar AM, Kader AA. 2004. Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) fruits. J Agric Food Chem 52:5694–5701
Defilippi BG, Kader AA, Dandekar AM. 2005. Apple aroma: alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci 168:1199–1210
Dopico B, Lowe AL, Wilson ID, Merodio C, Grierson D. 1993. Cloning and characterization of avocado fruit mRNAs and their expression during ripening and low-temperature storage. Plant Mol Biol 21:437–449
El-Kereamy A, Chervin C, Roustan JP, Cheynier V, Souquet JM, Moutounet M, Raynal J, Ford C, Latche A, Pech JC, Bouzayen M. 2003. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol Plant 119:175–182
Fan XT, Mattheis JP, Fellman JK. 1998. A role for jasmonates in climacteric fruit ripening. Planta 204:444–449
Fei ZJ, Tang X, Alba RM, White JA, Ronning CM, Martin GB, Tanksley SD, Giovannoni JJ. 2004. Comprehensive EST analysis of tomato and comparative genomics of fruit ripening. Plant J 40:47–59
Fei ZJ, Tang XM, Alba R, Giovannoni J. 2006. Tomato Expression Database (TED): a suite of data presentation and analysis tools. Nucleic Acids Res 34:D766–D770
Flores F, El Yahyaoui F, de Billerbeck G, Romojaro F, Latche A, Bouzayen M, Pech JC, Ambid C. 2002. Role of ethylene in the biosynthetic pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J Exp Bot 53:201–206
Flores FB, Martinez-Madrid M, Ben Amor M, Pech JC, Latche A, Romojaro F. 2004. Modified atmosphere packaging confers additional chilling tolerance on ethylene-inhibited cantaloupe Charentais melon fruit. European Food Res Technol 219:614–619
Fox AJ, Del Pozo-Insfran D, Lee JH, Sargent SA, Talcott ST. 2005. Ripening-induced chemical and antioxidant changes in bell peppers as affected by harvest maturity and postharvest ethylene exposure. Hortscience 40:732–736
Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D. 2004. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305:1786–1789
Frye CA, Tang DZ, Innes RW. 2001. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci U S A 98:373–378
Gagne J-M, Smalle J, Gingerich D-J, Walker J-M, Yoo S-D, Yanagisawa S, Vierstra R-D. 2004. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci U S A 101:6803–6808
Gao Z, Chen Y-F, Randlett MD, Zhao X-C, Findell JL, Kieber JJ, Schaller GE. 2003. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278:34725–34732
Giovannoni JJ. 2004. Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180
Given NK, Venis MA, Grierson D. 1988. Hormonal regulation of ripening in the strawberry a non-climacteric fruit. Planta 174:402–406
Goldschmidt EE, Huberman M, Goren R. 1993. Probing the role of endogenous ethylene in the degreening of citrus-fruit with ethylene antagonists. J Plant Growth Regul 12:325–329
Griffiths A, Barry C, Alpuche-Solis AG, Grierson D. 1999. Ethylene and developmental signals regulate expression of lipoxygenase genes during tomato fruit ripening. J Exp Bot 50:793–798
Grumet R, Fobes JF, Herner RC. 1981. Ripening behavior of wild tomato species. Plant Physiol 68:1428–1432
Guis M, Botondi R, BenAmor M, Ayub R, Bouzayen M, Pech JC, Latche A. 1997. Ripening-associated biochemical traits of Cantaloupe Charentais melons expressing an antisense ACC oxidase transgene. J Am Soc Horticult Sci 122:748–751
Guo H, Ecker JR. 2003. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell 115:667–677
Guo H, Ecker JR. 2004. The ethylene signaling pathway: New insights. Curr Opin Plant Biol 7:40–49
Gur A, Semel Y, Cahaner A, Zamir D. 2004. Real time QTL of complex phenotypes in tomato interspecific introgression lines. Trends Plant Sci 9:107–109
Gussman CD, Goffreda JC, Gianfagna TJ. 1993. Ethylene production and fruit-softening rates in several apple fruit ripening variants. Hortscience 28:135–137
Gutterson N, Reuber TL. 2004. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7:465–471
Guzman P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-Related mutants. Plant Cell 2:513–524
Hackett RM, Ho C-W, Lin Z, Foote HCC, Fray RG, Grierson D. 2000. Antisense inhibition of the Nr gene restores normal ripening to the tomato Never-ripe mutant, consistent with the ethylene receptor-inhibition model. Plant Physiol 124:1079–1085
Haji T, Yaegaki H, Yamaguchi M. 2001. Changes in ethylene production and flesh firmness of melting, nonmelting and stony hard peaches after harvest. J Jpn Soc Horticult Sci 70:458–459
Haji T, Yaegaki H, Yamaguchi M. 2003. Softening of stony hard peach by ethylene and the induction of endogenous ethylene by 1-aminocyclopropane-1-carboxylic acid (ACC). J Jpn Soc Horticult Sci 72:212–217
Hall AE, Chen QG, Findell JL, Schaller GE, Bleecker AB. 1999. The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121:291–299
Harada T, Sunako T, Wakasa Y, Soejima J, Satoh T, Niizeki M. 2000. An allele of the 1-aminocyclopropane-1-carboxylate synthase gene (Md-ACS1) accounts for the low level of ethylene production in climacteric fruits of some apple cultivars. Theoret Appl Genet 101:742–746
Hirschberg J. 2001. Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218
Hobson GE, Nichols R, Davies JN, Atkey PT. 1984. The inhibition of tomato lycopersicon-esculentum fruit ripening by silver. J Plant Physiol 116:21–30
Hua J, Meyerowitz EM. 1998. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94:261–271
Iannetta PPM, Laarhoven LJ, Medina-Escobar N, James EK, McManus MT, Davies HV, Harren FJM. 2006. Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiol Plant 127:247–259
Jacob-Wilk D, Holland D, Goldschmidt EE, Riov J, Eyal Y. 1999. Chlorophyll breakdown by chlorophyllase: isolation and functional expression of the Chlase1 gene from ethylene-treated citrus fruit and its regulation during development. Plant J 20:653–661
Jarret RL, Tigchelaar EC, Handa AK. 1984. Ripening behavior of the green-ripe tomato lycopersicon-esculentum mutant. J Am Soc Horticult Sci 109:712–717
Katz E, Lagunes PM, Riov J, Weiss D, Goldschmidt EE. 2004. Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta 219:243–252
Kerr EA. 1958. Mutations of chlorophyll retention in ripe fruit. Rep Tomato Genet Coop 8:22
Kerr EA. 1982. Never ripe-2 (Nr-2) a slow ripening mutant resembling Nr and Gr. Rep Tomato Genet Coop 32:33
Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. 1993. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72:427–441
Klee HJ. 2004. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiol 135:660–667
Klee HJ, Hayford MB, Kretzmer KA, Barry GF, Kishore GM. 1991. Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 3:1187–1194
Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. 1994. The never ripe mutation blocks ethylene perception in tomato. Plant Cell 6:521–530
Lashbrook CC, Tieman DM, Klee HJ. 1998. Differential regulation of the tomato ETR gene family throughout plant development. Plant J 15:243–252
Leclercq J, Adams-Phillips LC, Zegzouti H, Jones B, Latche A, Giovannoni JJ, Pech J-C, Bouzayen M. 2002. LeCTR1, a tomato CTR1-like gene, demonstrates ethylene signaling ability in Arabidopsis and novel expression patterns in tomato. Plant Physiol 130:1132–1142
Lelievre JM, Latche A, Jones B, Bouzayen M, Pech JC. 1997. Ethylene and fruit ripening. Physiol Plant 101:727–739
Lin Z, Hackett RM, Payton S, Grierson D. 1998. A tomato sequence, TCTR2 (accession no. AJ005077), encoding an Arabidopsis CTR1 homologue. Plant Physiol 117:1126
Lincoln JE, Fischer RL. 1988. Regulation of gene-expression by ethylene in wild-type and rin tomato (lycopersicon-esculentum) fruit. Plant Physiol 88:370–374
Lincoln JE, Cordes S, Read E, Fischer RL. 1987. Regulation of gene expression by ethylene during lycopersicon-esculentum tomato fruit development. Proc Natl Acad Sci U S A 84:2793–2797
Litt A, Irish VF. 2003. Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: Implications for the evolution of floral development. Genetics 165:821–833
Liu Y, Schiff M, Dinesh-Kumar S-P. 2002. Virus-induced gene silencing in tomato. Plant J 31:777–786
Liu YS, Gur A, Ronen G, Causse M, Damidaux R, Buret M, Hirschberg J, Zamir D. 2003. There is more to tomato fruit colour than candidate carotenoid genes. Plant Biotechnol J 1:195–207
Malcomber ST, Kellogg EA. 2005. SEPALLATA gene diversification: brave new whorls. Trends Plant Sci 10:427–435
Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB. 2006. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38:948–952
Marty I, Douat C, Tichit L, Jungsup K, Leustek T, Albagnac G. 2000. The cystathionine-gamma-synthase gene involved in methionine biosynthesis is highly expressed and auxin-repressed during wild strawberry (Fragaria vesca L.) fruit ripening. Theoret Appl Genet 100:1129–1136
Matarasso N, Schuster S, Avni A. 2005. A novel plant cysteine protease has a dual function as a regulator of 1-aminocyclopropane-1-carboxylic acid synthase gene expression. Plant Cell 17:1205–1216
McMurchie EJ, McGlasson WB, Eaks IL. 1972. Treatment of fruit with propylene gives information about biogenesis of ethylene. Nature 237:235–236
Moeder W, Barry CS, Tauriainen AA, Betz C, Tuomainen J, Utriainen M, Grierson D, Sandermann H, Langebartels C, Kangasjarvi J. 2002. Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol 130:1918–1926
Mueller LA, Tanksley SD, Giovannoni JJ, van Eck J, Stack S, Choi D, Kim BD, Chen MS, Cheng ZK, Li CY, Ling HQ, Xue YB, Seymour G, Bishop G, Bryan G, Sharma R, Khurana J, Tyagi A, Chattopadhyay D, Singh NK, Stiekema W, Lindhout P, Jesse T, Lankhorst RK, Bouzayen M, Shibata D, Tabata S, Granell A, Botella MA, Giullano G, Frusciante L, Causse M, Zamir D. 2005. The Tomato Sequencing Project, the first cornerstone of the International Solanaceae Project (SOL). Comp Funct Genomics 6:153–158
Nakano R, Ogura E, Kubo Y, Inaba A. 2003. Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiol 131:276–286
Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. 1998. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118:1295–1305
O’Malley RC, Rodriguez FI, Esch JJ, Binder BM, O’Donnell P, Klee HJ, Bleecker AB. 2005. Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J 41:651–659
Oeller PW, Min Wong L, Taylor LP, Pike DA, Theologis A. 1991. Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254:437–439
Oetiker JH, Olson DC, Shiu O-Y, Yang SF. 1997. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum). Plant Mol Biol 34:275–286
Olmedo G, Guo HW, Gregory BD, Nourizadeh SD, Aguilar-Henonin L, Li HJ, An FY, Guzman P, Ecker JR. 2006. ETHYLENE-INSENSITIVE5 encodes a 5′ → 3′ exoribonuclease required for regulation of the EIN3-targeting F-box proteins EBF1/2. Proc Natl Acad Sci U S A 103:13286–13293
Oraguzie NC, Iwanami H, Soejima J, Harada T, Hall A. 2004. Inheritance of the Md-ACS1 gene and its relationship to fruit softening in apple (Malus x domestica Borkh.). Theoret Appl Genet 108:1526–1533
Payton S, Fray RG, Brown S, Grierson D. 1996. Ethylene receptor expression is regulated during fruit ripening, flower senescence and abscission. Plant Mol Biol 31:1227–1231
Pedraza-Lopez A, Blanco-Portales R, Lopez-Raez J, Medina-Escobar N, Munoz-Blanco J, Rodriguez A (2006) Characterization of a strawberry late-expressed and fruit-specific peptide methionine sulphoxide reductase. Physiol Plant 126:129-139
Perin C, Gomez-Jimenez M, Hagen L, Dogimont C, Pech J-C, Latche A, Pitrat M, Lelievre J-M. 2002. Molecular and genetic characterization of a non-climacteric phenotype in melon reveals two loci conferring altered ethylene response in fruit. Plant Physiol 129:300–309
Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D. 1993a. Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J 3:469–481
Picton S, Gray J, Barton S, Abubakar U, Lowe A, Grierson D. 1993b. CDNA cloning and characterisation of novel ripening-related mRNAs with altered patterns of accumulation in the ripening inhibitor (rin) tomato ripening mutant. Plant Mol Biol 23:193–207
Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. 2003. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115:679–689
Purvis AC, Barmore CR. 1981. Involvement of ethylene in chlorophyll degradation in peel of citrus-fruits. Plant Physiol 68:854–856
Resnick JS, Wen C-K, Shockey JA, Chang C. 2006. REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc Natl Acad Sci USA 103:7917–7922
Rick CM. 1956. New mutants. Rep Tomato Genet Coop 6:22–23
Rose JKC, Bennett AB. 1999. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci 4:176–183
Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1991. 1 Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol 222:937–962
Sato T, Kudo T, Akada T, Wakasa Y, Niizeki M, Harada T. 2004. Allelotype of a ripening-specific 1-aminocyclopropane-1-carboxylate synthase gene defines the rate of fruit drop in apple. J Am Soc Horticult Sci 129:32–36
Schauer N, Semel Y, Roessner U, Gur A, Balbo I, Carrari F, Pleban T, Perez-Melis A, Bruedigam C, Kopka J, Willmitzer L, Zamir D, Fernie AR. 2006. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat Biotechnol 24:447–454
Sisler EC. 2006. The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnol Adv 24:357–367
Slater A, Maunders MJ, Edwards K, Schuch W, Grierson D. 1985. Isolation and characterization of complementary DNA clones for tomato Lycopersicon-esculentum cultivar Ailsa-Craig polygalacturonase and other ripening-related proteins. Plant Mol Biol 5:137–148
Solano R, Stepanova A, Chao Q, Ecker JR. 1998. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ethylene-insensitive3 and ethylene-response-factor1. Genes Dev 2:3703–3714
Stewart I, Wheaton TA. 1972. Carotenoids in citrus—their accumulation induced by ethylene. J Agric Food Chem 20:448–449
Sunako R, Sakuraba W, Senda M, Akada S, Ishikawa R, Niizeki M, Harada T. 1999. An allele of the ripening-specific 1-aminocyclopropane-1-carboxylic acid synthase gene (ACS1) in apple fruit with a long storage life. Plant Physiol 119:1297–1303
Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR. 2006. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol 140:150–158
Tang DZ, Christiansen KM, Innes RW. 2005. Regulation of plant disease resistance, stress responses, cell death, and ethylene signaling in Arabidopsis by the EDR1 protein kinase. Plant Physiol 138:1018–1026
Tatsuki M, Haji T, Yamaguchi M. 2006. The involvement of 1-aminocyclopropane-1-carboxylic acid synthase isogene, Pp-ACS1, in peach fruit softening. J Exp Bot 57:1281–1289
Tesniere C, Pradal M, El-Kereamy A, Torregrosa L, Chatelet P, Roustan JP, Chervin C. 2004. Involvement of ethylene signalling in a non-climacteric fruit: new elements regarding the regulation of ADH expression in grapevine. J Exp Bot 55:2235–2240
Thompson AJ, Tor M, Barry CS, Vrebalov J, Orfila C, Jarvis MC, Giovannoni JJ, Grierson D, Seymour GB. 1999. Molecular and genetic characterization of a novel pleiotropic tomato-ripening mutant. Plant Physiol 120:383–389
Tieman DM, Klee HJ. 1999. Differential expression of two novel members of the tomato ethylene-receptor family. Plant Physiol 120:165–172
Tieman DM, Taylor MG, Ciardi JA, Klee HJ. 2000. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci U S A 97:5663–5668
Tieman DM, Ciardi JA, Taylor MG, Klee HJ. 2001. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26:47–58
Tieman D, Taylor M, Schauer N, Fernie AR, Hanson AD, Klee HJ. 2006. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc Natl Acad Sci U S A 103:8287–8292
Tigchelaar EC, McGlasson WB, Buescher RW. 1978. Genetic regulation of tomato fruit ripening. HortScience 13:508–513
Tournier B, Sanchez-Ballesta M-T, Jones B, Pesquet E, Regad F, Latche A, Pech J-C, Bouzayen M. 2003. New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett 550:149–154
Trainotti L, Pavanello A, Casadoro G. 2005. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J Exp Bot 56:2037–2046
Van-der-Hoeven R, Ronning C, Giovannoni J, Martin G, Tanksley S. 2002. Deductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing. Plant Cell 14:1441–1456
Vardhini BV, Rao SSR. 2002. Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61:843–847
Villavicencio L, Blankenship SM, Sanders DC, Swallow WH. 1999. Ethylene and carbon dioxide production in detached fruit of selected pepper cultivars. J Am Soc Horticult Sci 124:402–406
Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. 2002. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296:343–346
Watkins CB. 2006. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol Adv 24:389–409
Whitelaw CA, Lyssenko NN, Chen L, Zhou D, Mattoo AK, Tucker ML. 2002. Delayed abscission and shorter internodes correlate with a reduction in the ethylene receptor LeETR1 transcript in transgenic tomato. Plant Physiol 128:978–987
Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. 1997. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15:444–447
Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. 1995. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science 270:1807–1809
Yanagisawa S, Yoo S-D, Sheen J. 2003. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425:521–525
Yang SF, Hoffman NE. 1984. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–190
Yen H-C, Lee S, Tanksley SD, Lanahan MB, Klee HJ, Giovannoni JJ. 1995. The tomato Never-ripe locus regulates ethylene-inducible gene expression and is linked to a homolog of the Arabidopsis ETR1 gene. Plant Physiol 107:1343–1353
Yokotani N, Tamura S, Nakano R, Inaba A, Kubo Y. 2003. Characterization of a novel tomato EIN3-like gene (LeEIL4). J Exp Bot 54:2775–2776
Yokotani N, Tamura S, Nakano R, Inaba A, McGlasson WB, Kubo Y. 2004. Comparison of ethylene- and wound-induced responses in fruit of wild-type, rin and nor tomatoes. Postharvest Biol Technol 32:247–252
Zarembinski TI, Theologis A. 1994. Ethylene biosynthesis and action: A case of conservation. Plant Mol Biol 26:1579–1597
Zegzouti H, Jones B, Frasse P, Marty C, Maitre B, Latche A, Pech J-C, Bouzayen M. 1999. Ethylene-regulated gene expression in tomato fruit: Characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J 18:589–600
Zheng XY, Wolff DW. 2000. Ethylene production, shelf-life and evidence of RFLP polymorphisms linked to ethylene genes in melon (Cucumis melo L.). Theoret Appl Genet 101:613–624
Zhou D, Kalaitzis P, Mattoo AK, Tucker ML. 1996. The mRNA for an ETR1 homologue in tomato is constitutively expressed in vegetative and reproductive tissues. Plant Mol Biol 30:1331–1338
Acknowledgments
Work highlighted in this review, which was performed by C.B. and others in the Giovannoni laboratory, was supported by the USDA-NCGRI (2002-35304-12530), National Science Foundation (DBI-0501778, DBI- 0605659), and the United States Department of Agriculture – Agricultural Research Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barry, C.S., Giovannoni, J.J. Ethylene and Fruit Ripening. J Plant Growth Regul 26, 143–159 (2007). https://doi.org/10.1007/s00344-007-9002-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-007-9002-y