Abstract
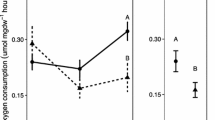
Oceanic uptake of anthropogenic carbon dioxide results in a decrease in seawater pH, a process known as “ocean acidification”. The pearl oyster Pinctada fucata, the noble scallop Chlamys nobilis, and the green-lipped mussel Perna viridis are species of economic and ecological importance along the southern coast of China. We evaluated the effects of seawater acidification on clearance, respiration, and excretion rates in these three species. The ammals were reared in seawater at pH 8.1 (control), 7.7, or 7.4. The clearance rate was highest at pH 7.7 for P. fucata and at pH 8.1 for C. nobilis and P. viridis. The pH had little effect on the respiration rate of P. fucata and P. viridis. In contrast, the respiration rate was significantly lower at pH 7.4 in C. nobilis. The excretion rate was significantly lower at pH 7.4 than pH 8.1 for all species. The results indicate that the reduction in seawater pH likely affected the metabolic process (food intake, oxygen consumption, and ammonia excretion) of these bivalves. Different species respond differently to seawater acidification. Further studies are needed to demonstrate the exact mechamsms for this effect and evaluate adaptability of these bivalves to future acidified oceans.
Similar content being viewed by others
References
Berg S J, Fisher S, Landrum P F. 1996. Clearance and processing of algal particles by zebra mussels (Dreissena polymorpha). J. Great Lakes Res., 22: 779–788.
Berge JA, Bjerkeng B, Pettersen O, Schanmng M T, Oxnevad S. 2006. Effects of mcreased sea water concentrations of CO2 on growth of the bivalve Mytilus edulis L. Chemosphere, 62: 681–687.
Bibby R, Widdicombe S, Parry H, Spicer J I, Pipe R. 2008. Impact of ocean acidification on the immune response of the blue mussel Mytilus edulis. Aquat. Biol., 2: 67–74.
Caldeira K, Wickert M E. 2003. Anthropogenic carbon and ocean pH. Nature, 425: 365–368.
Caldeira K, Wickert M E. 2005. Ocean model prediction of chemistry changes from carbon dioxide emission to the atmosphere and ocean. Geophys. Res. Lett., 110: C09S04. http://dx.doi.org/10.1029/2004JC002671.
Choi M C, Hsieh D P H, Lam P K S, Wang W X. 2003. Field depuration and biotransformation of paralytic shellfish toxins in scallop Chlamys nobilis and green-lipped mussel Perna viridis. Mar. Biol., 143: 927–934.
Comeau S, Gorsky G, Jeffree R, Teyssié J L, Gattuso J P. 2009. Impact of ocean acidification on a key Arctic pelagic mollusc (Limacina helicina). Biogeosciences, 6: 1 877–1882.
Czemy J, Barcelos e Ramos J, Riebesell U. 2009. Influence of elevated CO2 concentrations on cell division and nitrogen fixation rates in the bloom-forming cyanobactenum Nodularia spumigena. Biogeosciences, 6: 1 865–1 875.
Doney S C, Fabry V J, Feely RA. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci., 1: 169–192.
Dupont S, Thorndyke M. 2009. Impact of CO2-dnven ocean acidification on invertebrates early life-history—What we know, what we need to know and what we can do. Biogeosciences Discuss., 6: 3 109–3 131.
Dupont S, Thorndyke M. 2008. Ocean acidification and its impact on the early life-history stages of marine animals, In: Impact of Acidification on Biological, Chemical and Physical Systems in the Mediterranean and Black Seas, CIESM, Mongraph. p. 89–97.
Feely RA, Sabine C L, Lee K, Berelson W, Kleypas J, Fabry V J, Millero F J. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 305: 362–366.
Fmdlay H S, Wood H L, Kendall M A, Spicer J I, Twitchett R J, Widdicombe S. 2009. Calcification, a physiological process to be considered in the context of the whole organism. Biogeosciences Discuss., 6: 2 267–2 284.
Gazeau F, Quibher C, Jansen J M, Gattuso J P, Middelburg J J, Heip C H R. 2007. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett., 34: L07603, http://dx.doi.org/10.1029/2006GL028554.
Grasshoff K, Erhardt M, Kremling K. 1983. Methods of Seawater Analysis. Verlag Chemie, Weinheim, Germany.
Gumotte J M, Fabry V J. 2008. Ocean acidification and its potential effects on marine ecosystems. Ann. New York Acad. Sci., 1 134: 320–342.
Gutierrez J L, Jones C G, Strayer D L, Libarne O O. 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos, 101: 79–90.
Havenhand JN, Schlegel P. 2009. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences Discuss., 6: 4 573–4 586.
He M X, Huang L M, Shi J H, Jiang Y P. 2005. Variability of nbosomal DNA ITS-2 and its utility m detecting genetic relatedness of pearl oyster. Mar. Biotechnol., 15: 40–45.
IPCC (Intergovernmental Panel on Climate Change). 2007. Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z et al. eds. Contribution of Working Group 1 to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Jørgensen C B. 1990. Bivalve filter feeding: Hydrodynamics, Bioenergetics, Physiology and Ecology. Olsen & Olsen. p. 140.
Kurrhara H. 2008. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser., 373: 275–284.
Kurrhara H, Asai T, Kato S, Ishrmatsu A. 2008. Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis. Aquat. Biol., 4: 225–233.
Kurrhara H, Kato S, Ishimatsu A. 2007. Effects of increased seawater pCO2 on the early development of the oyster Crassostrea gigas. Aquat. Bio., 1: 91–98.
Loayza-Muro R, Elias-Letts R. 2007. Responses of the mussel Anodontites trapesialis (Unionidae) to environmental stressors: effect of pH, temperature and metals on filtration rate. Environ. Pollut., 149: 209–215.
Mao Y Z, Zhou Y, Yang H S, Wang R C. 2006. Seasonal vanation in metabolism of cultured Pacific oyster, Crassostrea gigas, in Sanggou Bay, China. Aquaculture, 253: 322–333.
Michaehdis B, Ouzoums C, Paieras A, Pörtner H O. 2005. Effects of long-term moderate hypercapma on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser., 293: 109–118.
Nagarajan R, Lea S E G, Goss-Custard J D. 2006. Seasonal variations in mussel Mytilus edulis L. shell thickness and strength and their ecological implications. J. Exp. Mar. Biol. Ecol., 339: 241–250.
Orr J C, Fabry V J, Aumont O, Bopp L et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437: 681–686.
Sabine C, Feely R A, Gruber N, Key R M et al. 2004. The oceanic sink for anthropogenic CO2. Science, 305: 367–371.
Saucedo P E, Ocampo L, Monteforte M, Bervera H. 2004. Effect of temperature on oxygen consumption and ammonia excretion m the Calafia mother-of-pearl oyster, Pinctada mazatlanica (Hanley, 1856). Aquaculture, 229: 377–387.
Sylvester F, Dorado J, Boltovskoy D, Juarez A, Cataldo D. 2005. Filtration rates of the invasive pest bivalve Limnoperna fortunei as a function of size and temperature. Hydrobiologia, 534: 71–80.
Todgham A E, Hofmann G E. 2009. Transcnptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J. Exp. Biol., 212: 2 579–2 594.
Widdicombe S, Needham H R. 2007. Impact of CO2-induced seawater acidification on the burrowing activity of Nereis virensand sediment nutrient flux. Mar. Ecol. Prog. Ser., 341: 111–122.
Wood H L, Spicer J I, Widdicombe S. 2008. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B, 275: 1 767–1 773.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (No. 41006090), the Knowledge Innovation Program of Chinese Academy of Sciences (No. KZCX2-YW-Q07-03), and the National High Technology Research and Development Program of China (863 Program) (No. 2006AA10A409)
Rights and permissions
About this article
Cite this article
Liu, W., He, M. Effects of ocean acidification on the metabolic rates of three species of bivalve from southern coast of China. Chin. J. Ocean. Limnol. 30, 206–211 (2012). https://doi.org/10.1007/s00343-012-1067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-012-1067-1