Abstract
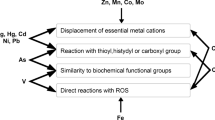
The antioxidant enzyme activity and malondialdehyde (MDA) content of Cephalothrix hongkongiensis were studied to assess variations in the biochemical/physiological parameters of nemerteans under heavy metal stress. Worms were exposed to copper, zinc and cadmium solutions at different concentrations, and the activity of three antioxidant enzymes, catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX), and MDA content were measured. The results show that the activity of each enzyme changed immediately after exposure to heavy metals. CAT was invariably inhibited throughout the experimental period, while the SOD activity was significantly elevated by exposure to Cu2+ for 48 h, but then decreased. SOD was inhibited by Zn2+during the first 12 h of exposure, but activated when exposed for longer periods. Under Cd2+ stress, SOD activity decreased within 72 h. GPX activity varied greatly, being significantly increased by both Cu2+ and Zn2+, but significantly inhibited by Cd2+ in the first 12–24 h after exposure. MDA content increased on Cu2+ exposure, but normally decreased on Zn2+ exposure. MDA content followed an increase-decrease-increase pattern under Cd2+ stress. In conclusion, the antioxidant system of this nemertean is sensitive to heavy metals, and its CAT activity may be a potential biomarker for monitoring heavy metal levels in the environment.
Similar content being viewed by others
References
Almeida E A, de Miyamoto S, Bainy A C D, de Medeiros M H G, Mascio P D. 2004. Protective effect of phospholipid hydroperoxide glutathione peroxidase (PHGPx) against lipid peroxidation in mussels Perna perna exposed to different metals. Marine Pollution Bulletin, 49: 386–392.
Bagnyukova T V, Storey K B, Lushchak V I. 2005. Adaptive response of antioxidant enzymes to catalase inhibition by aminotriazole in goldfish liver and kidney. Comparative Biochemistry and Physiology Part B, 142: 335–341.
Bagnyukova T V, Chahrak O I, Lushchak V I. 2006. Coordinated response of goldfish antioxidant defenses to environmental stress. Aquatic Toxicology, 78: 325–331.
Barja de Quiroga G, López-Torres M, Pérez-Campo R. 1989. Catalase is needed to avoid tissue peroxidation in Rana perezi in normoxia. Comparative Biochemistry and Physiology Part C, 94: 391–398.
Beuge J A, Aust S D. 1978. Microsomal lipids peroxidation. Methods in Enzymology, 52: 302–310.
Bouzyk E, Iwanenko T, Jarocewicz N, Kruszewski M, Sochanowicz B, Szumiel I. 1997. Antioxidant defense system in differentially hydrogen peroxide sensitive L5178Y sublines. Free Radical Biology and Medicine, 22: 697–704.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72: 248–254.
Casalino E, Calzaretti G, Sblano C, Landriscina C. 2002. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology, 179: 37–50.
Chandran R, Sivakuma A A, Mohandas S, Aruchami M. 2005. Effect of cadmium and zinc on antioxidant enzyme activity in the gastropod, Achatina fulica. Comparative Biochemistry and Physiology Part C, 140: 422–426.
Correia A D, Livingstone D R, Costa M H. 2002. Effects of water-borne copper on metallothionein and lipid peroxidation in the marine amphipod (Gammarus locusta). Marine Environmental Research, 54: 357–360.
Dorval J, Hontela A. 2003. Role of glutathione redox cycle and catalase in defense against oxidative stress induced by endosulfan in adrenocortical cells of rainbow trout (Oncorhynchus mykiss). Toxicology and Applied Pharmacology, 192: 191–200.
Drevet J R. 2006. The antioxidant glutathione peroxidase family and spermatozoa: A complex story. Molecular and Cellular Endocrinology, 250: 70–79.
Formigari A, Irato P, Santon A. 2007. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: Biochemical and cytochemical aspects. Comparative Biochemistry and Physiology Part C, 146: 443–459.
Geret F, Serafim A, Barreira L, Bebianno M J. 2002. Response of antioxidant systems to copper in the gills of the clam Ruditapes decussates. Marine Environmental Research, 54: 413–417.
Géret F, Jouan A, Turpin V, Bebianno M J, Cosson R P. 2002. Influence of metal exposure on metallothionein synthesis and lipid peroxidation in two bivalve mollusks: the oyster (Crassostrea gigas) and the mussel (Mytilus edulis). Aquatic Living Resources, 15: 61–66.
Góth L. 1991. A simple method for determination of serum catalase activity, and revision of reference range. Clinica Chimica Acta, 196: 143–152.
Harris E D. 1992. Regulation of antioxidant enzymes. The FASEB Journal, 6: 2 675–2 683.
Hafeman D G, Sunde R A, Hoekstra W G. 1974. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. Journal of Nutrition, 104: 580–587.
Kim G B, Lee R F. 2004. Effects of genotoxic compounds on DNA and development of early and late grass shrimp embryo stages. Marine Environmental Research, 57: 329–338.
Kono Y, Fridovich I. 1982. Superoxide radical inhibits catalase. The Journal of Biological Chemistry, 257: 5 751–5 764.
Kucukbay Z, Yazlak H, Sahin N, Tuzcu M, Cakmak M N, Gurdogan F, Juturu V, Sahin K. 2006. Zinc picolinate supplementation decreases oxidative stress in rainbow trout (Oncorhynchus mykiss). Aquaculture, 257: 465–469.
Lopes P A, Pinheiro T, Santos M C, Mathias M D L, Collares-Pereia M J, Viegas-Crespo A M. 2001. Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) toinorganic pollutants exposure. The Science of the Total Environment, 280: 153–163.
McCord J M, Fridovich I. 1969. Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). The Journal of Biological Chemistry, 244: 6 049–6 055.
McEvoy E G. 1988. Heavy metals in marine nemerteans. Hydrobiologia, 156: 135–143.
Michiels C, Raes M, Toussaint O, Remacle J. 1994. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radical Biology and Medicine, 17: 235–248.
Palace V P, Mjewski H S, Klaverkamp J F. 1992. Interactions among antioxidant defenses in liver of rainbow trout (Oncorhyncus mykiss) exposed to cadmium. Canadian Journal of Fisheries and Aquatic Sciences, 50: 156–162.
Pan L Q, Zhang H X. 2006. Metallothionein, antioxidant enzymes and DNA strand breaks as biomarkers of Cd exposure in a marine crab, Charybdis japonica. Comparative Biochemistry and Physiology Part C, 144: 65–75.
Prakash T N, Rao K S J. 1995. Modulations in antioxidant enzymes in different tissues of marine bivalve Perna viridis during heavy metal exposure. Molecular and Cellular Biochemistry, 46: 107–113.
Radi A A, Matkovics B. 1988. Effects of metal ions on the antioxidant enzyme activity, protein contents and lipid peroxidation of Carp tissues. Comparative Biochemistry and Physiology Part C, 90: 69–72.
Regoli F, Winston G W. 1998. Application of a new method for measuring the total oxyradical scavenging capacity in marine invertebrates. Marine Environmental Research, 46: 439–442.
Roméo M, Gnassia-Barelli M. 1997. Effect of heavy metals on lipid peroxidation in the Mediterranean clam Ruditapes decussatus. Comparative Biochemistry and Physiology Part C, 118: 33–37.
Santon A, Formigari A, Albergoni V, Irato P, 2006. Effect of Zn treatment on wild type and MT-null cell lines in relation to apoptotic and/or necrotic processes and on MT isoform gene expression. Biochimica et Biophysica Acta, 1 763(3): 305–312.
Shaheen A A, Abd El-Fattah A A. 1995. Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. The International Journal of Biochemistry & Cell Biology, 27: 89–95.
Sridevi B, Reddy K V, Reddy S L N. 1998. Effect of trivalent and hexavalent chromium on antioxidant enzyme activity and lipid peroxidation in a freshwater field crab, Barytelphusa guerini. Bulletin of Environmental Contamination and Toxicology, 61: 384–390.
Stebbing A R D. 1982. Hormesis-the stimulation of growth by low levels of inhibition. The Science of the Total Environment, 22: 212–234.
Valko M, Rhodes C J, Moncol J, Izakovic M, Mazur M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions, 160: 1–40.
Viarengo A, Canesi L, Pertica M, Poli G, Moore M N, Orunesu M. 1990. Heavy metal effects on lipid peroxidation in the tissues of Mytilus galloprovincialis Lam. Comparative Biochemistry and Physiology Part C, 97: 37–42.
Winston G W, Di Guilio R J. 1991. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquatic Toxicology, 19: 137–161.
Zikic R V, Stajn A, Saicic Z, Spasic M, Ziemnicki K, Petrovic V. 1996. The activity of superoxide dismutase, catalase and ascorbic acid content in the liver of goldfish (Carassius auratus gibelio Bloch.) exposed to cadmium. Physiological Research, 45: 479–481.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 30270235)
Rights and permissions
About this article
Cite this article
Wu, H., Zhao, X. & Sun, S. Variations of antioxidant enzyme activity and malondialdehyde content in nemertean Cephalothrix hongkongiensis after exposure to heavy metals. Chin. J. Ocean. Limnol. 28, 917–923 (2010). https://doi.org/10.1007/s00343-010-9050-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-010-9050-1