Abstract
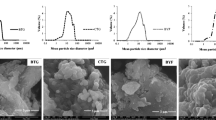
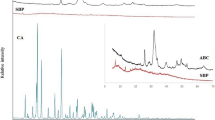
To investigate the possible use of waste products obtained after processing haddock, the present study prepared haddock bone calcium powder by NaOH and ethanol soaking (alkalinealcohol method) and prepared haddock bone calcium tablets using the powder in combination with appropriate excipients. The biological efficacy of the haddock bone calcium tablets was investigated using Wistar rats as an experiment model. Results show that the optimal parameters for the alkalinealcohol method are: NaOH concentration 1 mol/L, immersion time 30 h; ethanol concentration 60%, immersion time 15 h. A mixture of 2% polyvinylpyrrolidone in ethanol was used as an excipient at a ratio of 1:2 to full-cream milk powder, without the use of a disintegrating agent. This process provided satisfactory tablets in terms of rigidity and taste. Animal studies showed that the haddock bone calcium tablets at a dose of 2 g·kg−1·d−1 or 5g·kg−1·d−1 significantly increased blood calcium and phosphorus levels and bone calcium content in rats. Therefore, these tablets could be used for calcium supplementation and prevent osteoporosis. Although the reasons of high absorption in the rats fed with haddock bone calcium tablets are unclear, it is suggested that there are some factors, such as treatment with method of alkaline-alcohol or the added milk, may play positive roles in increasing absorption ratio.
Similar content being viewed by others
References
Deng S G, Peng Z Y, Yang P et al. 2001. Application of multi-enzymatic method in fermented fish sauce production from Harengula zumasi’s offal. J. Food and Fermentation Industries., 8(2):32–36. (in Chinese with English abstract)
Huang W K. 1989. Food Inspection and Analysis. Light Industry Press, Beijing, China. p. 238–245. (in Chinese)
Shi X Y. 1980. Method of Medical Animal Experiment. Publisher of People’s Hygiene, Beijing, China. p. 440–445. (in Chinese)
Mortensen L, Charles P. 1996. Bioavailability of calcium supplement and the effect of vitamin D: comparisons between milk, calcium carbonate, and calcium carbonate plus vitamin D. J. Am. So.c Clin. Nutr., 63: 354–357.
Lu H M. 2004. Continuous lactic acid fermentation of Haddock hydrolysate. J. Wuxi University of Light Industry, 23(6):103–106. (in Chinese with English abstract)
Mi S Q, Zhao H X, Jiang S. 2008. Study oncalcium absorptivity in calcium supplements evaluated by 41Ca labeled calcium pool of osteoporotic rats. J. Acta Nutirmenta Scinica., 30(1): 39–42. (in Chinese with English abstract)
Nancy F K. 2000. Bioavailability of dietary supplements and impact of physiologic state: infants, children and adolescents. J. Nutr., 131: 1 351–1 352.
Pan W S, Chen W D, Zeng X H. 1996. The preparation and stability of compound active calcium tablets. Chinese Journal of Hospital Pharmacy, 31(8): 474–477. (in Chinese with English abstract)
Qian D M, Shen Q G, Ke L R. 1994. Biological assay of calcitonin by blood calcium determination in rats. Chinese Journal of Pharmaceutical, 14(3): 30–34. (in Chinese with English abstract)
Sheikh M S, Ana C A S, Nicar M J et al. 1988. Gastrointestinal absorption of calcium from milk and calcium salts. J. New Engl. Med., 317: 532–536.
Tsugawa N, Okano T, Higashino R et al. 1995. Bioavailability of calcium from calcium carbonate, DL-calcium lactate, L-calcium lactate and powdered oyster shell calcium in vitamin D-deficient or replete rats. Biol. Pharm. Bull., 18: 677–682.
Wang J Z, Deng F R, Zhu L R. 1994. Utilization of the head and bone of freshwater fishes. J. Food Science, 2: 47–50. (in Chinese with English abstract)
Wu Q H, Gu W W, Lin H J. 2005. Introduction to a method of collecting blood from rat. J. Progress In Veterinary Medicine, 25(1): 133. (in Chinese)
Wu Y Y, Li L H, Lin H et al. 2005. Studies on preparation technology of CMC actived calcium and its bioavailability from Tilapia bone. J. Food Science., 26(2): 114–117. (in Chinese with English abstract)
Xue C H, Li J Z, Sun C et al. 1995. Studies on the preparation of active Calcium from pollack frame. J. Qingdao Ocean University, 25(2): 173–178. (in Chinese with English abstract)
Xu S G. 1996. Calcium powder of freshwater fish bone. J. Shanghai Fisheries University, 5(4): 246–251. (in Chinese with English abstract)
Yu J, Chen M Z. 2000. Study on preparation and application of hydrolyzed eel head protein by enzymatic methods. Chinese Journal of Marine Drugs, 19(5): 50–66. (in Chinese with English abstract)
Zhao X H, Mi Q S, Liu Y. 2006. Bioavailability study of two marketing calcium supplements on growing rat. J. Food Science, 27(10): 521–524. (in Chinese with English abstract)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Major Technology Project of Zhejiang Province (No. 2007C12013).
Rights and permissions
About this article
Cite this article
Huo, J., Deng, S., Xie, C. et al. Preparation and biological efficacy of haddock bone calcium tablets. Chin. J. Ocean. Limnol. 28, 371–378 (2010). https://doi.org/10.1007/s00343-010-9019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-010-9019-0