Abstract
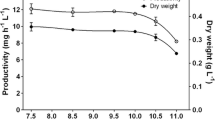
The unicellular cyanobacterium Synechocystis sp. PCC 6803, a model organism known for its unique combination of highly desirable molecular genetic, physiological and morphological characteristics, was employed in the present study. The species was cultured in BG11 liquid medium contained various initial concentrations of Pb2+ and Cd2+ (0, 0.5, 1, 2, 4, 6 and 8 mg/L). The experiment was conducted for six days and the metal induced alterations in the ultrastructure, growth and pigment contents were assessed. Alterations in the ultrastructure of the Synechocystis sp. PCC 6803 cells became evident with the increased (>4 mg/L Pb2+) metal concentration. The photosynthetic apparatus (thylakoid membranes) were found to be the worst affected. Deteriorated or completely destroyed thylakoid membranes have made large empty spaces in the cell interior. In addition, at the highest concentration (8 mg/L Pb2+), the polyphosphate granules became more prominent both in size and number. Despite the initial slight stimulations (0.2, 3.8 and 6.5% respectively at 0.5, 1 and 2 mg/L Pb2+), both metals inhibited the growth in a dose-dependent manner as incubation progressed. Pigment contents (chlorophyll α, β carotene and phycocyanin) were also decreased with increasing metal concentration. Cells exposed to 6 mg/L Pb2+, resulted in 36.56, 37.39 and 29.34% reductions of chlorophyll α, β carotene and phycocyanin respectively over the control. Corresponding reductions for the same Cd2+concentrations were 57.83, 48.94 and 56.90%. Lethal concentration (96 h LC50) values (3.47 mg/L Cd2+ and 12.11 mg/L Pb2+) indicated that Synechocystis sp. PCC 6803 is more vulnerable to Cd2+ than Pb2+.
Similar content being viewed by others
References
Ari, A. B., M. Mel, M. A. Hasan and M. I. A. Karim, 1999. The kinetics and mechanism of lead (II) biosorption by powderized Rhizopus oligosporus. World J. Microbiol. Biotechnol. 15: 291–298.
Atici, T., S. Ahiska, A. Altinda and D. Aydin, 2008. Ecological effects of some heavy metals (Cd, Pb, Hg, Cr) pollution of phytoplanktonic algae and zooplanktonic organisms in Sarıyar Dam Reservoir in Turkey. African J. Biotech. 7(12): 1 972–1 977.
Atri, N. and L. C. Rai, 2003. Differential responses of three cyanobacteria to UV-B and Cd. J. Microbiol. Biotech. 13: 544–551.
Beale, S. I., 1994. Biosynthesis of cyanobacterial tetrapyrrole pigments: hemes, chlorophylls and phycobilins. In: Bryant, D. A. ed. The Molecular Biology of Cyanobacteria. Kluwer Academic Publishers, Dordrecht. p. 519–558.
Bennert, A. and L. Bogorad, 1973. Complementary chromatic adaptation in filamentous blue-green algae. J. Cell Biol. 58: 419–435.
Bwn-Amotz, A. and M. Avron, 1983. On the factors which determine massive 3-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 72: 593–597.
Fathi, A. A., 2002. Toxicological response of the green alga Scenedesmus bijuga to mercury and lead. Folia Microbiol. 47: 667–671.
Fargasova, A., 1999. The green alga Scenedesmus quadricauda-a subject for the study of inhibitory effects of Cd, Cu, Zn, Pb and Fe. Biologia 54: 393–398.
Goudey, J. S., 1987. Modeling the inhibitory effects of metals on phytoplankton growth. Aqua. Toxicol. 10: 265–278.
Heng, L. Y., K. Jusoh, C. H. M. Ling and M. Idris, 2004. Toxicity of single and combinations of lead and cadmium to the cyanobacteria Anabaena flos-aquae. Bull. Environ. Contam. Toxicol. 72: 373–379.
Ismail, M., S. M. Phang, S. L. Tong and M. T. Brown, 2002. A modified toxicity testing method using tropical marine microalgae. Environ. Monit. Assess. 75: 145–154.
Javed, M. and G. Mahmood, 2000. Studies on the metal tocxxicity of plankton in the river Ravi. Pak. J Biol. Sci. 3(12): 2 165–2 168.
Jensen, T. E., M. Baxter, J. W. Rachlin and B. Warkentine, 1982. Uptake of heavy metals by Plectonema boryanum (Cyanophyceae) into cellular components, especially polyphosphate bodies: an X-ray energy dispersive study. Environ. Pollut. 27: 119–127.
Lamaia, C., M. Kruatrachuea, P. Pokethitiyooka, E. S. Upathamb and V. Soonthornsarathoola, 2005. Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta: A laboratory study. Science Asia 31: 121–127.
Leborans, G. F. and A. Novillo, 1996. Toxicity and bioaccumulation of cadmium in Olishodiscus luteus (Raphidophyceae). Water Res. 30: 57–62.
Liberton, M., R. Howard Berg, J. Heuser, R. Roth and H. B. Pakrasi, 2006. Ultrastructure of the membrane systems in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Protoplasma 227: 129–138.
Navarro, L., M. Torres-Marquez, S. Gonzalez-Moreno, S. Devars and R. Hernandez, 1997. Comparison of physiological changes in Euglena gracilis during exposure to heavy metals of heterotrophic and autotrophic cells. Comp. Biochem. Physiol. 116: 265–272.
Pahlsson, A. M. B., 1989. Toxicity of heavy metals (Zn, Cd, Cu, Pb) to vascular plants. Water Air Soil Pollut. 47: 287–319.
Pettersson, A., L. Hällbom and B. Bergman, 1988. Aluminium effects on uptake and metabolism of phosphorus by the cyanobacterium Anabaena cylindrical. Plant Physiol. 86: 112–116.
Rangsayatorn, N., E. S. Upatham, M. Kruatrachue, P. Pokethitiyook and G. R. Lanza, 2002. Phytoremediation potential of Spirulina (Arthrospira) platensis: Biosorption and toxicity studies of cadmium. Environ. Pollut. 119: 45–53.
Sanita-di-Toppi, L. S. and R. Gabbrielli, 1999. Response to cadmium in higher plants. Environ. Exp. Bot. 41: 105–130.
Satoha, A., L. Q. Vudikariab, N. Kuranoa and S. Miyachia, 2005. Evaluation of the sensitivity of marine microalgal strains to the heavy metals, Cu, As, Sb, Pb and Cd. Environ. Intern. 31: 713–722.
Sobhan, R. and S. P. K. Sternberg, 1999. Cadmium removal using Cladophora. J. Environ. Sci. Health Part A 34: 53–72.
Vermass, W., 1996. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: Principles and possible biotechnology applications. J. Appl. Phycol. 8: 263–273.
Wang, T. C., J. C. Weissman, G. Ramesh, R. Varadarajan and J. R. Benemann, 1998. Heavy metal binding and removal by Phormidium. Bull. Environ. Contam. Toxicol. 60: 739–744.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Chinese Scholarship Council
Rights and permissions
About this article
Cite this article
Arunakumara, K.K.I.U., Zhang, X. Effects of heavy metals (Pb2+ and Cd2+) on the ultrastructure, growth and pigment contents of the unicellular cyanobacterium Synechocystis sp. PCC 6803. Chin. J. Ocean. Limnol. 27, 383–388 (2009). https://doi.org/10.1007/s00343-009-9123-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-009-9123-1