Abstract
In this article, the capping action of Sodium carboxymethyl cellulose (NaCMC) on ZnS nanoparticles synthesized by microwave-assisted method is reported. The X-ray diffraction and electron diffraction studies corroborated nanocrystalline nature of cubic ZnS. Discontinuous cluster growth of particles was implied by optical absorption studies. This was reflected in transmission electron microscope images. Fourier-transform infrared studies confirmed NaCMC capping on the surface of ZnS nanoparticles. Effective encapsulation of ZnS nanoparticles by NaCMC was further supported by Raman scattering, thermal studies and photoluminescence studies. Surface modification of ZnS nanoparticles with NaCMC appear to be suitable for specific biomedical applications due to the nontoxicity of ZnS and NaCMC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnS belongs to II–VI semiconductors with a wide band gap of 3.68 eV for sphalerite bulk phase. In addition to its low production cost and non-toxicity, ZnS is a thermally and chemically stable compound possessing a high dielectric constant of 8.7 [1]. Consequently, nanostructured ZnS finds itself in various fields such as super capacitors [2], solar energy applications [3], optical devices such as LED [4], biomedical applications [5][5] and luminescent display systems [7]. However, the nanosized ZnS particles are highly unstable and tend to agglomerate due to the surface effects of smallness in size. Therefore, confining the growth of nanoparticles (NPs) and providing surface passivation are of prime importance in the nanoparticle research. Biodegradable biopolymers offer an excellent opportunity in this direction.
Among naturally occurring biopolymers cellulose is most abundant one. Its derivatives have several advantages such as recyclability, high viscosity, nontoxicity, biodegradability and cost effectiveness [8]. Sodium carboxy methyl cellulose (NaCMC) is a water-soluble cellulose derivative with carboxy methyl groups attached to some of hydroxyl groups of glucopyranose monomers of cellulose backbone. NaCMC has received ample scientific attention, due to its polyelectrolyte character [9]. In view of its high transparency, good film-forming property, large mechanical strength, non-toxicity and biodegradability, it is found suitable for applications such as medicine, flocculating agent, chelating agent, emulsifier, thickening agent, water-retaining agent, sizing agent, and film-forming material [10,11,12]. Recent reports on NaCMC reveal that it works as a stabilizer in the preparation of certain semiconducting nanosized materials like PbO nanoparticles [13], ZnO [14], CdS [15], ZnS [16] and some magnetic nanoparticles such as Fe3O4 [17, 18]. Increase in light emission intensity has been reported in ref [16]. for NaCMC stabilisation of ZnS films.
A detailed study of preparation of NaCMC-capped ZnS NPs and characterization is relatively less explored. In the present work, we report a novel synthesis approach for ZnS nanostructures using microwave-assisted aqueous precipitation technique. Microwave heating is a simple, fast and efficient method in which material heating takes place by dielectric heating [19]. In conventional heating, an external thermal source heats the reaction medium by a conductive heating. But microwave irradiation (MWI) causes efficient in-core volumetric heating by direct coupling of microwave (MW) energy with the molecules of solvents, reagents, catalysts, etc. that are present in the reaction mixture [19]. The quick and uniform heating of reaction mixture in MW heating induces fast homogeneous nucleation that leads to the formation of uniform sized small NPs [19], while prolonged hydrothermal conventional heating leads to form bigger particles with better crystallinity [20]. MW-assisted synthesis leads to the formation of more defects in the synthesized powders [21]. In unpassivated MW-synthesized nanoparticles, such defects on surfaces diminish the luminous intensity in them [22]. MW-assisted chemical reactions performed at the optimized reaction temperatures are found to be cleaner, produce less biproducts compared to conventionally heated processes [23].
Zinc acetate dihydrate (ZA) and thioacetamide (TA) were used as precursors for ZnS and NaCMC was used as the capping agent. The synthesis involves non-toxic precursors, solvent (water) and energy-saving principles of microwave heating such that most of the principles of green chemistry are followed. Very good photoluminescence behavior was exhibited by the properly capped sample.
2 Experimental
2.1 Chemicals
Zinc acetate dihydrate [Zn(CH3COO)2. 2H2O)], thioacetamide (CH3CSNH2) and sodium carboxy methyl cellulose (NaCMC) were purchased from Alfa Aesar (Germany). All chemical reagents were of pure analytical grade and used without further purification. Double distilled (DD) water was used as solvent.
2.2 Synthesis of samples
The synthesis procedure followed in the present work is similar to that explained in ref [24]. and [4] with certain modifications. About 0.025 g of NaCMC was dissolved in DD water and aged for 24 h. To 0.15 M zinc acetate aqueous solution, NaCMC solution was added and mixed well by stirring. Thioacetamide (TA) solution at a concentration of 0.27 M in water was added to this. Total volume of the mixture was maintained to be 100 ml. The mixture was subjected to microwave irradiation in a domestic microwave oven (LG model: MS-2049UW of 20 L capacity operating at frequency 2.45 GHz) for predetermined 6 min. The reaction was evidenced by a change in the color from clear to milky white. After cooling, ZnS was separated from solution by centrifugation. Sample was dried and named as S1. Experiment was repeated for different amounts like 0.05, 0.075 and 0.1 wt % of NaCMC addition and samples were named as S2, S3 and S4, respectively. At concentrations beyond 0.1 wt%, the solution was so viscous that collection of particles became difficult and the obtained sample was sticky. Pure sample of ZnS was also synthesized without the addition of NaCMC for comparison.
2.3 Characterization
X-ray diffraction (XRD) was carried out with Rigaku Miniflex 600 X-ray diffractometer with monochromatic copper–Kα radiation of wavelength 1.5402 Å. TEM analyses were done by JOEL JEM 2100 high-resolution transmission electron microscope (HRTEM) and UV–Vis absorption studies were carried out using SHIMADZU – 1800PC spectrophotometer for the dilute dispersion of sample in DD water. The ZnS powders were dispersed in KBr pellets and FTIR spectra was obtained with NICOLET– 6700 spectrophotometer. Photoluminescence (PL) spectra were obtained with Hitachi F-7000 Fluorescence Spectrophotometer. Raman scattering experiments were performed using BRUKER RFS 27:Stand-alone FT-Raman Spectrometer. The thermal analysis was done using the thermal analysis instrument SDT Q600 V20.9 Build 20 in nitrogen atmosphere at a heating rate of 3ºK/min for pure ZnS, ZnS-NaCMC composite S2 and neat NaCMC.
3 Results and discussion
3.1 X-ray diffraction
XRD patterns of ZnS-NaCMC nanocomposites and pure ZnS are shown in Fig. 1. The diffraction peaks are observed for 2 \(\uptheta\) values at 28º, 48º and 57º. The peaks are identified to originate from (111), (220) and (311) planes of the zinc blende structure of ZnS (JCPDS No. 01-072-4841). No peaks for other phases or elements are observed showing high purity of samples and the broad diffraction peaks is an indication of the particles in the nanometer dimension. The average nanocrystallite size (\(\mathrm{D}\)) is determined for the prominent peak (111) using Scherrer equation:
where \(\uplambda\) is wavelength of incident X-rays; \(\theta\) is angle of diffraction, β is full width at half maximum (FWHM) of Bragg peak. The NaCMC capped samples show slight increase in crystallite size compared to uncapped ZnS. Murugadoss et al. explained similar increase in crystallite size due to binding of surfactant on the ZnS nanoparticles [25]. But the nanocrystallites do not appear to change much in size with NaCMC concentrations. Interplanar separations (dhkl) between the planes (hkl) are calculated using Braggs’ law and lattice parameter ‘\(a\)’ for cubic lattice is determined using:
From the X-ray diffractogram, we have estimated degree of crystallinity using the ratio of area under crystalline peaks to the total area of crystalline peaks and amorphous regions [26]. The variation of nanocrystallite size, lattice parameter and degree of crystallinity for pure and NaCMC added ZnS samples are given in Table 1. Figure 2 depicts the variation of lattice constant and degree of crystallinity with NaCMC concentration. Lattice parameter is found to decrease with concentration of NaCMC, reaches minimum in the case of S2, and increases thereafter. The crystallinity increases with increase in concentration of NaCMC till 0.05% (S2) and decreases for further increase in concentration indicating good crystallinity for the sample S2. The improvement in crystallinity with NaCMC concentration is attributed to decrease in defects number due to the capping action of NaCMC, resulting in less imperfection in the crystallites. This continues up to 0.05 wt% concentration of NaCMC and thereafter crystallinity deceases due to aggregation of nanoparticles, seen in TEM micrograph.
3.2 UV–Vis spectroscopy
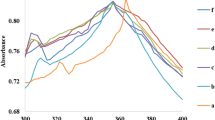
The plot of absorbance versus wavelength for all samples is shown in Fig. 3.
The excitonic absorption occurs at ~ 322 nm for pure ZnS while it occurs at ~ 332 nm for NaCMC-capped ZnS samples. These values are less than that for bulk counterpart which occurs at ~ 337 nm indicating that the particles are in nano-regime [28]. The appearance of sharp absorption maximum in Fig. 4 shows the monodisperse nature of nanoparticles [29]. With increasing concentration of NaCMC, the position of the absorption maximum remains almost same. This implies that nanoparticle growth is taking place not through Ostwald ripening, for, Ostwald ripening is recognized by the continuation of the excitonic absorption band towards larger wavelengths [30]. Instead, a well-defined absorption maximum occurs repeatedly, implying a discontinuous cluster growth of ZnS nanocrystals has taken place due to the anionic surfactant NaCMC. From XRD studies also, we observe that the size of coated NPs do not change much for the capped samples except for 0.1% NaCMC. The UV absorption curves show some absorption in the visible region and this is attributed to defect related states in the band gap [31].
From the studies above, it is apparent that sample S2 is better stabilized among the capped samples and this sample is selected for further analysis. To investigate about morphology, TEM and HRTEM images were taken for ZnS-pure, sample S2 and S4.
3.3 Electron microscopy
TEM and HRTEM images of ZnS-pure, samples S2 and S4 are shown.
TEM image shown in Fig. 4a for uncapped ZnS indicates the formation of solid spheres of size ~ 100 nm. The bigger size is due to the absence of capping agent. The lattice image (Fig. 4b) for ZnS-pure sample shows crystalline nature which is supported by SAED pattern. The SAED patterns in all cases indicate the polycrystalline behavior with phases (111), (220) and (311) of cubic ZnS.
In the case of sample S2, formation of spherical nanoclusters of near uniform size is shown by Fig. 5a. Each of these clusters can be seen to contain dispersed primary nanoparticles separated by a thin polymer layer.
In the HRTEM image of the coated nanoparticles (Fig. 5b), an ultrathin film of NaCMC polymer is clearly seen around ZnS nanoparticles and this film is bound intact to the nanoparticles. Good crystallinity of the sample is exhibited in the lattice image of the micrograph which was revealed by XRD studies also. Discontinuous lattice fringes indicate the polycrystallinity of the sample [32]. The size of NPs is estimated from the HRTEM image (Fig. 5b) the mean size is about 2.6 nm, which is similar to that of XRD results. It has been observed in nanoparticle physics that semiconductor nanocrystals with crystallite size in the range of exciton Bohr radius show size dependent optical properties. The exciton Bohr radius for ZnS is about 2.5 nm.
Figure 6 shows the TEM image for sample S4. It reveals that, at high concentration of NaCMC, the ZnS NPs formed are aggregated in the matrix of NaCMC. However, the ZnS NPs formed in this case also exhibited cubic polycrystalline nature indicated by the diffraction pattern.
3.4 Fourier-transform infrared spectroscopy (FTIR)
FTIR is based on the principle that the chemical bonds can be excited at frequencies that are typical of the bonds and can provide valuable information about passivation of surface of nanoparticles by the polymer. Figure 7 shows the FTIR spectra for sample S2 and neat NaCMC. The broad absorption band at 3418 cm−1 for NaCMC corresponds to O–H stretching and the band at 2915 cm−1 corresponds to C-H stretching vibrations. The strong band at 1602 cm−1 and 1418 cm–1 are due to COO− group [33]. The band at 1325 cm−1 is assigned to -OH bending vibrations [34, 35]. The characteristic –C-O stretching on polysachcharide skeleton is indicated by the absorption band at 1056 cm−1. The band at 664 cm−1 is due to ZnS present in sample S2. The hydroxyl peaks of NaCMC shifted to lower wave number 3397 cm−1 for S2 indicating a strong interaction between hydroxyl groups of NaCMC and ZnS NPs [36]. The asymmetric and symmetric stretching vibrations at 2921 and 2849 cm−1 are due to –C–H vibrations and arise in S2 due to nanostructure formation [17, 37]. The band corresponding to COO– shift to 1565 cm−1. The presence of carboxyl and hydroxyl groups in the final ZnS-CMC compound is a strong confirmation that there is an interaction between zinc precursor and NaCMC molecule.
The growth of ZnS NPs and the capping action of polymer NaCMC may be understood from the chemical reactions involved in the synthesis. During synthesis, the Zn2+ ions formed in aqueous solution of zinc acetate make complexes with NaCMC added. This solution was microwave (MW) irradiated after the addition of thioacetamide (TA). MWI induces volumetric heating in the material due to inertial, elastic and frictional forces. The power absorbed by the material is represented by [38]:
where \(f\) is frequency (GHz), \({\varepsilon }_{0}\) is permittivity of free space,\({\varepsilon }_{r }^{^{\prime}}\) is relative dielectric constant, tan \(\delta\) is the loss factor and E is magnitude of internal field. As a result, MW irradiation affects the synthesis process in two ways. First, the polymer NaCMC decomposes during MWI to some extent and produce shorter chains which enable the Zn2+ ions to coordinate with –OH ligands or make bond with COO– groups [39]. Second, it accelerates the decomposition of TA to provide S2− ions as:
As soon as S2− ions become available, reaction between Zn2+ and S2− ions takes place to form ZnS nuclei. ZnS nuclei formed grow into nanoparticles under the combining force between Zn2+ ions and S2− ions within the cavities bound by the polymer chains [40]. NaCMC being anionic long chain polymer, provides electrostatic as well as steric stabilization. Hence, an increase in crystallite size is observed for NaCMC-capped samples compared to uncapped ZnS. At lower concentration of NaCMC, the availability of fewer –CH2COO– ligands attached to NaCMC lead to incomplete coverage of ZnS NPs. For concentrations of NaCMC like, 0.05% the NaCMC serves as a shell and final particles formed are nearly spherical, possess uniform size and can disperse well in water [11]. For higher concentrations like 0.1%, the space between polymer chains becomes narrower as a result of increased viscosity [32]. The increased number of polymer chains now provides more carboxyl and hydroxyl linkages to Zn2+ ions leading to the formation of large number of ZnS nuclei in a small space. Hence, the decrease in crystallinity observed with samples S3 and S4 compared to S2 may be attributed to the aggregation.
3.5 Photoluminescence (PL) spectroscopy
The influence of NaCMC capping on the photoluminescence (PL) of ZnS NPs is investigated and PL graphs are shown in Fig. 8. The samples were excited at 250 nm. The luminescence observed with ZnS- Pure and sample S2 indicate visible emission. Hence emission phenomenon involves localized states in the band gap. The PL spectra obtained are broad and asymmetric, consisting of more than one component. Gaussian curve fitting was applied to deconvolute the PL peaks. The best Gaussian fit gave five peaks for pure ZnS. Weak emission at 350 nm is attributed to S interstitials [IS] and peak at 363 nm is attributed to Zn interstitials [IZn].The strong peak centered at 391 nm is assigned to the recombination of electrons at the excess Zn present [41]. Peak at 442 nm is attributed to transition from surface sulfur vacancy [SVS]. Emission peak near 469 nm is due to internal S vacancy [VS] to internal Zn vacancy [VZn] [42].
In the case of NaCMC-capped sample, the peak at 355 nm is attributed to interstitial S [IS] ions. As the radius of S ions is larger than that of Zn ions, interstitial S causes greater strain and possess less binding energy. Corresponding energy levels lie close to valence band and energy levels for Zn interstitials lie close to conduction band. Zn-related emission occurs at 399 nm. Peak near 470 nm is assigned due to internal S and Zn vacancies. Surface sulfur vacancy related emissions are not observed for NaCMC capped sample.
Energy-dispersive X-ray analysis showed that the amount of sulfur is less compared to zinc in the capped as well as uncapped samples which may give rise to sulfur vacancies at the surface causing Zn dangling bonds and form shallow donor levels. But in the emission spectra of capped sample, no band corresponding to surface sulfur vacancy is found indicating the strong coupling of dangling bonds of ZnS with NaCMC. As supported by FTIR, there is bonding between NaCMC and excess Zn2+ ions present on surface of ZnS nanoparticles.
The important difference between PL spectra of pure ZnS and S2 is in the number of PL peaks and their intensity. In pure-ZnS, due to surface sulfur and surface zinc related 5 emission peaks were observed, but on capping, these emissions were absent. The capped ZnS is having more PL intensity owing to controlled homogeneous growth of the nanoparticles in solution medium. The higher PL intensity in NaCMC-capped sample is attributed to better passivation of surface-related defects, leading to an increase in electron–hole recombination [43, 44]. The low PL intensity observed with ZnS-Pure is due to the presence of surface defects which cause nonradiative decay.
Schematic of the photoemission in S2 is represented by Fig. 9.
3.6 Raman scattering
Raman spectroscopy is a fast and useful technique to analyze surface properties of nanosized particles. Figure 10 corresponds to the room temperature Raman spectra of sample S2 and neat NaCMC obtained using excitation source of wavelength 1024 nm. The excitation energy is in off-resonance regime for ZnS. Bands observed for capped ZnS nanoparticles near 260 cm−1 and 343 cm−1 are assigned to transverse optical (TO) and longitudinal optical (LO) modes, respectively, for cubic phase of ZnS. For bulk ZnS, these bands were reported to occur at 276 cm−1 for TO and 351 cm−1 for LO modes [45].The larger Raman shift from bulk in the present study may be due to the effect of phonon confinement resulting from the small size of particles (≈2.3 nm). Some of the skeletal and carboxylate ion related vibrations due to NaCMC also occur in S2. The assignment of Raman vibrations for neat NaCMC and S2 are derived from the literature [46,47,48] and given in Table 2. The appearance of Raman modes of vibration for NaCMC along with that for ZnS in sample S2 indicates the interaction of NaCMC with ZnS NPs.
There were no Raman bands around 286 cm−1 corresponding to wurtzite phase [45]. This is in accordance with XRD results which show peaks corresponding to cubic ZnS only.
3.7 Thermal properties
Thermo gravimetric analysis (TGA) plays an important role in determining thermal stability of the materials. To understand the thermal behavior of the ZnS-NaCMC composite, TGA were performed for ZnS-pure, sample S2 and neat NaCMC. TGA curves are shown in Fig. 11. In all curves, the initial loss in weight is due to the loss of adsorbed gases from the surface. For sample S2 and neat NaCMC, the second loss in weight occurs due to the loss of CO2 from the Cellulose. In this temperature range, the decarboxylation of COO− group in CMC takes place which demonstrates the decomposition of CMC [49]. Neat NaCMC experienced a 50% weight loss at a temperature of ∼300 °C, whereas for sample S2, it was only 15% weight loss at temperature ∼450 °C. The TGA curve for neat NaCMC is steep between 250ºC and 300ºC while sample S2 shows broader decomposition temperatures in the range 250ºC–450ºC, indicating a better thermal stability of S2 compared to NaCMC [50]. The drastic decrease in mass for pure ZnS and sample S2 beyond 600ºC indicates the decomposition of ZnS into its constituents.
Some of the properties of the sample S2 are presented in the following Table 3 along with the results obtained in other published works.
In all, we have seen that, the ZnS nanoparticles when properly capped with NaCMC, becomes a good light emitter. By studying magnetic nature of these NPs, this work can be extended for targeted drug delivery.
4 Conclusions
In summary, microwave assisted synthesis of NaCMC capped ZnS NPs was carried out by chemical precipitation method. From the studies on structural and optical properties of samples, following conclusions were drawn:
-
Powder XRD and TEM studies indicated that the synthesized samples of ZnS possessed sphalerite structure with average nanocrystallite size less than 3 nm. NaCMC-capped ZnS showed increase in nanocrystallite size compared to uncapped one. But it did not increase with concentration of NaCMC which was supported by UV-absorption studies. Blue shift of UV–Vis absorption peaks indicated quantum size effect.
-
The surface shallow defect related emissions of ZnS were quenched with NaCMC capping. A very good enhancement in photoluminescence intensity was observed with NaCMC-capped sample compared to uncapped ZnS.
-
FTIR and Raman studies suggested that capping action of NaCMC onto ZnS takes place through the linkages of –OH and COO— ligands.
-
The thermal studies also confirmed the interaction of NaCMC with ZnS.
References
M. Sabet, M. Salavati-Niasari, E. Esmaeili, Synthesis of zinc sulfide nanostructures with different sulfur sources via mild hydrothermal route: investigation of crystal phase and morphology. J. Inorg. Organomet. Polym. Mater. 26, 738–743 (2016). https://doi.org/10.1007/s10904-016-0374-y
R. Ramachandran, M. Saranya, P. Kollu, B.P.C. Raghupathy, S.K. Jeong, A.N. Grace, Solvothermal synthesis of zinc sulfide decorated graphene (ZnS/G) nanocomposites for novel supercapacitor electrodes. Electrochim. Acta. 178, 647–657 (2015). https://doi.org/10.1016/j.electacta.2015.08.010
R. Hernández Castillo, M. Acosta, I. Riech, G. Santana-Rodríguez, J. Mendez-Gamboa, C. Acosta, M. Zambrano, Study of ZnS/CdS structures for solar cells applications. Optik (Stuttg) 148, 95–100 (2017). https://doi.org/10.1016/j.ijleo.2017.09.002
S. Anjum, S. Shaheen, M.S. Awan, R. Zia, Effect of various surfactants on optical and electrical properties of Cu+2-doped ZnS semiconductor nanoparticles. Appl. Phys. A Mater. Sci. Process. (2019). https://doi.org/10.1007/s00339-019-2558-0
M.L. Desai, B. Deshmukh, N. Lenka, V. Haran, S. Jha, H. Basu, R.K. Singhal, P.F. Sharma, S.K. Kailasa, K.H. Kim, Influence of doping ion, capping agent and pH on the fluorescence properties of zinc sulfide quantum dots: Sensing of Cu2+ and Hg2+ ions and their biocompatibility with cancer and fungal cells, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 210, 212–221 (2019). https://doi.org/10.1016/j.saa.2018.11.027
H. Labiadh, K. Lahbib, S. Hidouri, S. Touil, T.B.E.N. Chaabane, Insight of ZnS nanoparticles contribution in different biological uses. Asian Pac. J. Trop. Med. 9, 757–762 (2016). https://doi.org/10.1016/j.apjtm.2016.06.008
C. Ramamoorthy, V. Rajendran, Formation of solid and hollow sphere ZnS nanoparticles by hydrothermal process and their structural, optical and photocatalytic activity. Appl. Phys. A. 124, 500 (2018). https://doi.org/10.1007/s00339-018-1851-7
M.S. Rani, N. Dzulkurnain, N.S. Ahmad, Mohamed, , Conductivity and dielectric behavior studies of carboxymethyl cellulose from kenaf bast fiber incorporated with ammonium acetate-BMATFSI biopolymer electrolytes. Int. J. Polym. Anal. Charact 20, 250–260 (2015). https://doi.org/10.1080/1023666X.2015.1013176
J.S. Behra, J. Mattsson, O.J. Cayre, E.S.J. Robles, H. Tang, T.N. Hunter, Characterization of sodium carboxymethyl cellulose aqueous solutions to support complex product formulation: a rheology and light scattering study. ACS Appl. Polym. Mater. 1, 344–358 (2019). https://doi.org/10.1021/acsapm.8b00110
A.C. De Jesus, S.M. Carvalho, L.C. De, H.S. Mansur, Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications SAP membrane superabsorbent crosslinked PEG-carboxymethyl cellulose tissue repaired hydrogels for potential treatment of wounds. Int. J. Biol. Macromol. (2017). https://doi.org/10.1016/j.ijbiomac.2017.08.124
M. Cui, F.J. Wang, Z.Q. Shao, F.S. Lu, W.J. Wang, Influence of DS of CMC on morphology and performance of magnetic microcapsules. Cellulose 18, 1265–1271 (2011). https://doi.org/10.1007/s10570-011-9570-7
T. Jiang, J. Li, L. Zhang, B. Wang, J. Zhou, Microwave assisted in situ synthesis of Ag-NaCMC films and their reproducible surface-enhanced Raman scattering signals. J. Alloys Compd. 602, 94–100 (2014). https://doi.org/10.1016/j.jallcom.2014.03.020
G. Mohammed, A.M. El Sayed, Preparation and characterization of PbO / carboxymethyl cellulose/polyvinylpyrrolidone nanocomposite films. Polym. Compos. (2017). https://doi.org/10.1002/pc.24402
P. Kanmani, J.W. Rhim, Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 106, 190–199 (2014). https://doi.org/10.1016/j.carbpol.2014.02.007
C.R. Tang, Z.H. Su, B.G. Lin, H.W. Huang, Y.L. Zeng, S. Li, H. Huang, Y.J. Wang, C.X. Li, G.L. Shen, R.Q. Yu, A novel method for iodate determination using cadmium sulfide quantum dots as fluorescence probes. Anal. Chim. Acta. 678, 203–207 (2010). https://doi.org/10.1016/j.aca.2010.08.034
J.F. Luna-Martínez, D.B. Hernández-Uresti, M.E. Reyes-Melo, C.A. Guerrero-Salazar, V.A. González-González, S. Sepúlveda-Guzmán, Synthesis and optical characterization of ZnS–sodium carboxymethyl cellulose nanocomposite films. Carbohydr. Polym. 84, 566–570 (2011). https://doi.org/10.1016/j.carbpol.2010.12.021
G. Aguilera, C.C. Berry, R.M. West, E. Gonzalez-Monterrubio, A. Angulo-Molina, Ó. Arias-Carrión, M.Á. Méndez-Rojas, Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood-brain barrier. Nanoscale Adv. 1, 671–685 (2019). https://doi.org/10.1039/c8na00010g
V.V. Spiridonov, I.G. Panova, L.A. Makarova, M.I. Afanasov, S.B. Zezin, A.V. Sybachin, A.A. Yaroslavov, The one-step synthesis of polymer-based magnetic γ-Fe2O3/carboxymethyl cellulose nanocomposites. Carbohydr. Polym. 177, 269–274 (2017). https://doi.org/10.1016/j.carbpol.2017.08.126
Y. Zhu, F. Chen, Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. (2013). https://doi.org/10.1021/cr400366s
S. Lee, D. Song, D. Kim, J. Lee, S. Kim, I.Y. Park, Y.D. Choi, Effects of synthesis temperature on particle size/shape and photoluminescence characteristics of ZnS: Cu nanocrystals. Mater. Lett. 58, 342–346 (2004). https://doi.org/10.1016/S0167-577X(03)00483-X
D.W. Synnott, M.K. Seery, S.J. Hinder, J. Colreavy, S.C. Pillai, Novel microwave assisted synthesis of ZnS nanomaterials. Nanotechnology. 24, 045704 (2013). https://doi.org/10.1088/0957-4484/24/4/045704
X. Liu, Z. Li, C. Zhao, W. Zhao, J. Yang, Y. Wang, F. Li, Facile synthesis of core-shell CuO/Ag nanowires with enhanced photocatalytic and enhancement in photocurrent. J. Colloid Interface Sci. 419, 9–16 (2014). https://doi.org/10.1016/j.jcis.2013.12.042
K. Patel, M.P.D.S.H. Chaki, Effect of Ag on structural, optical and luminescence properties of ZnS nanoparticles synthesized by microwave-assisted chemical route. Appl. Phys. A. 123, 367 (2017). https://doi.org/10.1007/s00339-017-0980-8
X. Tian, J. Wen, S. Wang, J. Hu, J. Li, H. Peng, Starch-assisted synthesis and optical properties of ZnS nanoparticles. Mater. Res. Bull. 77, 279–283 (2016). https://doi.org/10.1016/j.materresbull.2016.01.046
G. Murugadoss, Synthesis and optical characterization of PVP and SHMP-encapsulated Mn 2-doped ZnS nanocrystals. J. Lumin. 130, 2207–2214 (2010). https://doi.org/10.1016/j.jlumin.2010.06.021
P. Bansal, M. Hall, M.J. Realff, J.H. Lee, A.S. Bommarius, Multivariate statistical analysis of X-ray data from cellulose: A new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour. Technol. 101, 4461–4471 (2010). https://doi.org/10.1016/j.biortech.2010.01.068
P. Muhammed Shafi, A. Chandra Bose, Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 5, 057137 (2015). https://doi.org/10.1063/1.4921452
W. Sang, Y. Qian, J. Min, D. Li, L. Wang, Microstructural and optical properties of ZnS : Cu nanocrystals prepared by an ion complex transformation method. Solid. State. Communic. 121, 475–478 (2002). https://doi.org/10.1016/S0038-1098(01)00518-X
A.K. Kole, P. Kumbhakar, U. Chatterjee, Observation of nonlinear absorption and visible photoluminescence emission in chemically synthesized Cu2 + doped ZnS nanoparticles Observation of nonlinear absorption and visible photoluminescence emission in chemically synthesized Cu 2 1 doped ZnS nanoparticles. Appl. Phys. Lett. 100, 013103 (2012). https://doi.org/10.1063/1.3674307
S. Wageh, Z. Su, X. Xu-rong, Growth and optical properties of colloidal ZnS nanoparticles. J. Crystal Growth 255, 332–337 (2003). https://doi.org/10.1016/S0022-0248(03)01258-2
J. Zhang, S. Liu, J. Yu, M. Jaroniec, A simple cation exchange approach to Bi-doped ZnS hollow spheres with enhanced UV and visible-light photocatalytic H2-production activity. J. Mater. Chem. 21, 14655 (2011). https://doi.org/10.1039/c1jm12596f
J. Li, K. Inukai, Y. Takahashi, A. Tsuruta, W. Shin, Effect of PVP on the synthesis of high-dispersion core–shell barium-titanate–polyvinylpyrrolidone nanoparticles. J. Asian Ceram. Soc. 5, 216–225 (2017). https://doi.org/10.1016/j.jascer.2017.05.001
M.A. Martínez-Rodríguez, M.A. Garza-Navarro, I.E. Moreno-Cortez, R. Lucio-Porto, V.A. González-González, Silver/polysaccharide-based nanofibrous materials synthesized from green chemistry approach. Carbohydr. Polym. 136, 46–53 (2016). https://doi.org/10.1016/j.carbpol.2015.09.014
L.Y. Wang, M.J. Wang, Removal of heavy metal ions by Poly(vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze-thaw method. ACS Sustain. Chem. Eng. 4, 2830–2837 (2016). https://doi.org/10.1021/acssuschemeng.6b00336
D. de Britto, O.B.G. Assis, Thermal degradation of carboxymethylcellulose in different salty forms. Thermochim. Acta. 494, 115–122 (2009). https://doi.org/10.1016/j.tca.2009.04.028
J. Zeng, S. Liu, J. Cai, L. Zhang, TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. J. Phys. Chem. C. 114, 7806–7811 (2010). https://doi.org/10.1021/jp1005617
B.S.R. Devi, R. Raveendran, A.V. Vaidyan, Synthesis and characterization of Mn 2 + -doped ZnS nanoparticles. Pramana -J. Phys 68, 679–687 (2007)
S. Das, A.K. Mukhopadhyay, S. Datta, D. Basu, Prospects of microwave processing an overview. Bull Mater Sci. 31, 943–956 (2008). https://doi.org/10.1007/s12034-008-0150-x
N. Rangelova, L. Aleksandrov, T. Angelova, N. Georgieva, R. Müller, Preparation and characterization of SiO2/CMC/Ag hybrids with antibacterial properties. Carbohydr. Polym. 101, 1166–1175 (2014). https://doi.org/10.1016/j.carbpol.2013.10.041
Z. Zhou, D. He, W. Xu, F. Ren, Y. Qian, Preparing ZnS nanoparticles on the surface of carboxylic poly(vinyl alcohol) nanofibers. Mater. Lett. 61, 4500–4503 (2007). https://doi.org/10.1016/j.matlet.2007.02.041
N. Kumbhojkar, V.V. Nikesh, A. Kshirsagar, S. Mahamuni, N. Kumbhojkar, V.V. Nikesh, A. Kshirsagar, Photophysical properties of ZnS nanoclusters Photophysical properties of ZnS nanoclusters. J. Appl. Phys. 88, 6260 (2000). https://doi.org/10.1063/1.1321027
M. Sookhakian, Y.M. Amin, W.J. Basirun, M.T. Tajabadi, N. Kamarulzaman, Synthesis, structural, and optical properties of type-II ZnO – ZnS core – shell nanostructure. J. Lumin. 145, 244–252 (2014). https://doi.org/10.1016/j.jlumin.2013.07.032
S.J. Rosenthal, J.C. Chang, O. Kovtun, J.R. McBride, I.D. Tomlinson, Biocompatible quantum dots for biological applications. Chem. Biol. 18, 10–24 (2011). https://doi.org/10.1016/j.chembiol.2010.11.013
M. Taherian, A.A. Sabbagh Alvani, M.A. Shokrgozar, R. Salimi, S. Moosakhani, H. Sameie, F. Tabatabaee, Surface-treated biocompatible ZnS quantum dots: Synthesis, photo-physical and microstructural properties. Electron. Mater. Lett. 10, 393–400 (2014). https://doi.org/10.1007/s13391-013-3211-2
O. Brafman, S.S. Mitra, Raman effect in wurtzite- and zinc-blende-type ZnS single crystals. Phys. Rev. 171, 931–934 (1968). https://doi.org/10.1103/PhysRev.171.931
G. Socrates, in Biological Molecules - Macromolecules, ed. By Socretes, (John Wiley & Sons Ltd, England 2004) p. 329–330. https://doi.org/10.1002/jrs.1238.
P.J. Larkin, “IR and Raman Spectroscopy - Principles and Spectral Interpretation,” (Elsevier, Oxford, 2011) pp.135–176 https://doi.org/10.1016/b978-0-12-386984-5.10001-1.
J. Blackwell, P.D. Vasko, J.L. Koenig, Infrared and raman spectra of the cellulose from the cell wall of valonia ventricosa infrared and raman spectra of the cellulose from the cell wall of valonia ventricosa. J. Appl. Phys. 41, 4375 (2011). https://doi.org/10.1063/1.1658470
B. Sivakumar, R.G. Aswathy, Y. Nagaoka, M. Suzuki, T. Fukuda, Y. Yoshida, T. Maekawa, D.N. Sakthikumar, Multifunctional carboxymethyl cellulose-based magnetic nanovector as a theragnostic system for folate receptor targeted chemotherapy Imaging, and Hyperthermia against Cancer. Langmuir 29, 3453–3466 (2013). https://doi.org/10.1021/la305048m
M.N. Nadagouda, R.S. Varma, Synthesis of thermally stable carboxymethyl cellulose / metal biodegradable nanocomposites for potential biological applications. Biomacromol 8, 2762–2767 (2007). https://doi.org/10.1021/bm700446p
K. Hedayati, A. Zendehnam, F. Hassanpour, Fabrication and characterization of zinc sulfide nanoparticles and nanocomposites prepared via a simple chemical precipitation method. J Nanostruct. 6, 207–212 (2016). https://doi.org/10.7508/JNS.2016.03.005
G. Nabiyouni, R. Sahraei, M. Toghiany, M.H.M. Ara, K. Hedayati, Preparation and characterization of nanostructured ZnS thin films grown on glass and N-type Si substrates using a new chemical bath deposition technique. Rev. Adv. Mater. Sci. 27, 52–57 (2011)
F.A. La Porta, J. Andrés, M.S. Li, J.R. Sambrano, J.A. Varela, E. Longo, Zinc blende versus wurtzite ZnS nanoparticles: control of the phase and optical properties by tetrabutylammonium hydroxide. Phys. Chem. Chem. Phys. 16, 20127–37 (2014). https://doi.org/10.1039/c4cp02611j
S. Kumar, M. Singhal, J.K. Sharma, Functionalization and characterization of ZnS quantum dots using biocompatible l-cysteine. J. Mater. Sci. Mater. Electron. 24, 3875–3880 (2013). https://doi.org/10.1007/s10854-013-1332-x
Acknowledgements
The authors thank Bhandarkars’ Arts and Science college, Kundapura for sample preparation facilities, DST PURSE Laboratory, Mangalore University – Mangalore for thermal analysis, DST- SAIF centre, IIT Madras at Chennai for Raman scattering experiments, DST- SAIF centre at Karnatak University-Dharwad for FTIR and photoluminescence measurements and Manipal Academy of Higher Education—Manipal for other characterizations. Author Lalitha Devi B thanks the University Grants Commission, Government of India, for teacher fellowship.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Devi, B.L., Rao, K.M. & Ramananda, D. Spectroscopic investigation of green synthesized ZnS nanoparticles encapsulated by sodium carboxy methyl cellulose. Appl. Phys. A 126, 924 (2020). https://doi.org/10.1007/s00339-020-04107-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04107-y