Abstract
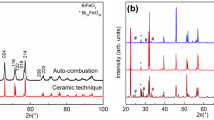
Perovskite LaCoO3 nanopowders were synthesized via solution auto-combustion chemical route, using lanthanum and cobalt nitrates as oxidants and citric acid as fuel. Qualitative and Quantitative phase analysis performed by Rietveld refinements indicated the presence of pure single LaCoO3 phase. From fit parameters, goodness of fit “S” parameter (close to 1) and the difference between the experimental and theoretical XRD patterns. FTIR spectra showed two characteristics bands at 408 and 589 cm−1 characteristics of metal oxygen bands vibration, thus confirming the formation of LaCoO3 phase, whereas the strong absorption band at 589 cm−1 was assigned to Co–O stretching vibration and O–Co–O deformation modes of LaCoO3 compound. SEM micrographs clearly revealed quite spherical particles with a mean size in the range 139–178 nm and high tendency to clustering, meanwhile EDAX analysis confirmed the corresponding chemical composition with homogeneous distribution of the constituent elements. A weak paramagnetic order is observed irrespective of F/O ratio, with slight increase of the effective magnetic moment with F/O ratio; 2.72–2.93 μB, due to the presence of more high spin states.





Similar content being viewed by others
References
A.S. Verma, V.K. Jindal, ABX3-type Oxides and Halides: Their Structure and Physical Properties (Nova Science Publishers, Hauppauge, New York, United States, 2004–2018), pp. 463–479
K. Asish, B. Kundu, Raveau, Structural, Magnetic and Electron Transport Properties of Ordered-Disordered Perovskite Cobaltites (Nova Science Publishers, Hauppauge, New York, United States, 2004–2018), pp. 213–250
Z. Yang, Y. Huang, B. Dong, H.L. Li, S.Q. Shi, Sol–gel template synthesis and characterization of LaCoO3 nanowires. Appl. Phys. A 84, 117–122 (2006)
S. Nakayam, M. Okazaki, Y.L. Aung, M. Sakamoto, Preparations of perovskite-type oxides LaCoO3 from three different methods and their evaluation by homogeneity, sinterability and conductivity. Solid State Ionics 158, 133–139 (2003)
Y. Zhu, R. Tan, T. Yi, S. Ji, X. Ye, L. Cao, Preparation of nanosized LaCoO3 perovskite oxide using amorphous heteronuclear complex as a precursor at low temperature. J. Mater. Sci. 35, 5415–5420 (2000)
D. Berger, V. Fruth, I. Jitaru, J. Schoonman, Synthesis and characterisation of La1−xSrxCoO3 with large surface area. Mater. Lett. 58, 2418–2422 (2004)
L. Amelao, G. Bandoli, D. Barreca, M. Bettinelli, G. Bottaro, A. Caneschi, Synthesis and characterization of nanophasic LaCoO3 powders. Surf. Interface Anal. 34, 112–115 (2002)
G. Sinquin, C. Petit, J.P. Hindermann, A. Kiennemann, Study of the formation of LaMO3 (M = Co, Mn) perovskites by propionates precursors: application to the catalytic destruction of chlorinated VOCs. Catal. Today 70, 183–196 (2001)
M. Popa, J. Frantti, M. Kakihana, Characterization of LaMeO3 (Me: Mn Co, Fe) perovskite powders obtained by polymerizable complex method. Solid State Ion. 154–155, 135–141 (2002)
T. Tatarchuk, A. Shyichuk, J. Lamkiewicz, J. Kowalik, Inversion degree, morphology and colorimetric parameters of cobalt aluminate nanopignments depending on reductant type in solution combustion synthesis. Ceram. Int. 46, 14674–14685 (2020)
T. Tatarchuk, M. Naushad, J. Tomaszewska, P. Kosobucki, M. Myslin, H. Vasylyeva, P. Scigalski, Adsorption of Sr(II) ions and salicylic acid onto magnetic magnesium-zinc ferrites: isotherms and kinetic studies. Environ. Sci. Pollut. Res. 27, 26681–26693 (2020)
J. Theerthagiri, G. Durai, T. Tatarchuk, M. Sumathi, P. Kuppusami, J. Qin, M.Y. Choi, Synthesis of hierarchial structured rare earth metal-doped Co3O4 by polymer combustion method for high performance electrochemical supercapacitor electrode materials. Ionics 26, 2051–2061 (2019)
K.C. Patil, S.T. Aruna, T. Mimani, Combustion synthesis: an update. Curr. Opin. Solid State Mater. Sci. 6, 507–512 (2002)
A.S. Mukasyan, C. Costello, K.P. Sherlock, D. Lafarga, A. Varma, Perovskite membranes by aqueous combustion synthesis: synthesis and properties. Sep. Purif. Technol. 25, 117–126 (2001)
T. Mimani, K.C. Patil, Solution combustion synthesis of nanoscale oxides and their composites. Mater. Phys. Mech. 4, 134–137 (2001)
D. Berger, C. Matei, Rev. Roum. Chim. 50, 889–894 (2005)
J.T. Antonio, S.T. Monica, J.A. Renato, B. Carlos, P. Bergmann, Synthesis by the solution combustion process and magnetic properties of iron oxide (Fe3O4 and α-Fe2O3) particles. J. Mater. Sci. 42, 4785–4791 (2007)
A.B. Salunkhe, V.M. Khot, M.R. Phadatare, S.H. Pawar, Combustion synthesis of cobalt ferrite nanoparticles—Influence of fuel to oxidizer ratio. J. Alloys Compd. 514, 91–96 (2012)
R. Elilarassi, G. Chandrasekaran, Synthesis and optical properties of Ni-doped zinc oxide nanoparticles for optoelectronic applications. Optoelectron. Lett. 6, 6–10 (2010)
D. Berger, N. van Landschoat, C. Ionica, F. Papa, V. Fruth, Synthesis of pure and doped lanthanum cobaltite by the combustion method. J. Optoelectron. Adv. Mater. 5, 719–724 (2003)
A. Barabauskas, D. Jasaitis, A. Kareiva, Characterization of sol-gel process in the Y–Ba–Cu–O acetate-tartrate system using IR spectroscopy. Vib. Spectrosc. 28, 263–275 (2002)
J. Chen, Y. Li, Y. Wang, J. Yun, D. Cao, Preparation and characterization of zinc sulfide nanoparticles under high-gravity environment. Mater. Res. Bull. 39, 185–194 (2004)
M.L. Dinesha, H.S. Jayanna, S. Ashoka, G.T. Chandrappa, Temperature dependent electrical conductivity of Fe doped ZnO nanoparticles prepared by solution combustion method. J. Alloy. Compd. 485, 538–541 (2009)
S. Zhou, L. Shi, J. Zhao, L. He, H. Yang, S. Zhang, Ferromagnetism in LaCoO3 nanoparticles. Phys. Rev. B 76, 172407 (2007)
Shiming Zhou, Laifa He, Shuangyi Zhao, Yuqiao Guo, Jiyin Zhao, Lei Shi, Size-dependent structural and magnetic properties of LaCoO3 nanoparticles. J. Phys. Chem. C 113, 13522–13526 (2009)
Yang Wang, Hong Jin Fan, Orbital ordering-driven ferromagnetism in LaCoO3 nanowires. J. Appl. Phys. 108, 053917 (2010)
C. Kittel, Introduction to solid state physics, Chap. 14 & 15, 7th edn. (Wiley, New York, 1996)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferby, V.A., Raj, A.M.E. & Bououdina, M. Dependence of structural/morphological and magnetic properties of LaCoO3 nanoparticles prepared by citrate nitrate auto combustion. Appl. Phys. A 126, 909 (2020). https://doi.org/10.1007/s00339-020-04085-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04085-1