Abstract
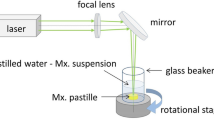
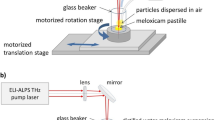
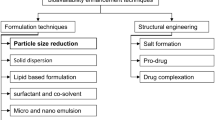
The laser fragmentation technique has been extensively used to produce inorganic nanoparticles, but its practice on organic materials, especially on drugs, is less common. Here, we briefly review the recent advances in laser micro-/nanonization of organic materials and the rationale of using laser fragmentation for drug discovery. We present our case studies of two drug models: fenofibrate and naproxen. Both drugs were fragmented in water with femtosecond (fs) laser and characterized in terms of particle size distribution and physicochemical properties. Effects of fs laser fragmentation were also compared with nanosecond (ns) laser fragmentation and with conventional media milling technique. Fs laser was more suitable to produce sub-micron size drug particles than ns laser, but degradation of drugs after nanonization was also more pronounced than micronization. Physicochemical transformations such as oxidation, hydration and amorphisation might occur during the laser–material interactions. Laser nanonization showed improved dissolution kinetics, similar to media milling. Unlike the conventional milling techniques, laser fragmentation enabled the treatment of minute amount (as small as several milligrams) of drugs with high efficiency, thus is a useful tool for particle size reduction during the early phases of drug discovery.






Similar content being viewed by others
References
S. Barcikowski, F. Devesa, K. Moldenhauer, J. Nanopart. Res. 11, 1883–1893 (2009)
S. Besner, M. Meunier, in Laser Precision Microfabrication, ed. by K. Sugioka, M. Meunier, A, Piqué (Springer, Berlin, 2010), pp. 163–187
S. Besner, A.V. Kabashin, M. Meunier, Appl. Phys. Lett. 89, 233122 (2006)
N.G. Semaltianos, Crit. Rev. Solid State Mater. Sci. 35, 105–124 (2010)
J.A. Dahl, B.L.S. Maddux, J.E. Hutchison, Chem. Rev. 107, 2228–2269 (2007)
G.W. Yang, Prog. Mater. Sci. 52, 648–698 (2007)
C.L. Sajti, R. Sattari, B.N. Chichkov, S. Barcikowski, J. Phys. Chem. C 114, 2421–2427 (2010)
Y. Tamaki, T. Asahi, H.J. Masuhara, Phys. Chem. A 106, 2135–2139 (2002)
T. Asahi, T. Sugiyama, H. Masuhara, Acc. Chem. Res. 41, 1790–1798 (2008)
H.-G. Jeon, T. Sugiyama, H. Masuhara, T. Asahi, J. Phys. Chem. C 111, 14658–14663 (2007)
J. Hobley, T. Nakamori, S. Kajimoto, M. Kasuya, K. Hatanaka, H. Fukumura, S.J. Nishio, Photochem. Photobiol. A 189, 105–113 (2007)
P. Wagener, S. Barcikowski, Appl. Phys. A 101, 435–439 (2010)
T. Sugiyama, S. Ryo, I. Oh, T. Asahi, H.J. Masuhara, Photochem. Photobiol. A 207, 7–12 (2009)
S. Kenth, J.-P. Sylvestre, K. Fuhrmann, M. Meunier, J.-C. Leroux, J. Pharm. Sci. 100, 1022–1030 (2011)
J.-P. Sylvestre, M.-C. Tang, A. Furtos, G. Leclair, M. Meunier, J.-C. Leroux, J. Control. Release 149, 273–280 (2011)
W. Ding, J.-P. Sylvestre, G. Leclair, M. Meunier, Int. J. Theo. Appl. Nanotech. 1, 99–104 (2012)
K. Taylor, in Pharmaceutics: The Design and Manufacture of Medicines, 3rd edn. ed. by M.E. Aulton (Churchill Livingstone, Edinburgh, 2007), pp. 539–554
T.A. Serajuddin, Adv. Drug Deliv. Rev. 59, 603–616 (2007)
M.E. Brewster, T. Loftsson, Adv. Drug Deliv. Rev. 341, 1–19 (2007)
T. Loftsson, M.E. Brewster, J. Pharm. Pharmacol. 62, 1607–1621 (2010)
J. Rautio, H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Jarvinen, J. Savolainen, Nat. Rev. Drug Discov. 7, 255–270 (2008)
R. Duncan, S. Gac-Breton, R. Keane, R. Musila, Y. Sat, R. Satchi, F. Searle, J. Control. Release 74, 135–146 (2001)
G. Bonacucina, M. Cespi, M. Misici-Falzi, G.F. Plamieri, J. Pharm. Sci. 98, 1–42 (2009)
V.S. Trubetskoy, V.P. Torchilin, Adv. Drug Deliv. Rev. 16, 311–320 (1995)
T.M. Allen, P.R. Cullis, Science 303, 1818–1822 (2004)
V.J. Stella, R.A. Rajewski, Pharm. Res. 14, 556–567 (1997)
I. Ghosh, W.M. Nau, Adv. Drug Deliv. Rev. 64, 764–783 (2012)
B.E. Rabinow, Nat. Rev. Drug Discov. 3, 785–796 (2004)
F. Kesisoglou, S. Panmai, Y. Wu, Adv. Drug Deliv. Rev. 59, 631–644 (2007)
N. Rasenack, H. Hartenhauer, B. Muller, Int. J. Pharm. 254, 137–145 (2003)
E. Merisko-Liversidge, G.G. Liversidge, E.R. Cooper, Eur. J. Pharm. Sci. 18, 113–120 (2003)
E. Reverchon, J. Supercrit. Fluids 15, 1–21 (1999)
J. Brouwers, M. Brewster, P. Augustijins, J. Pharm. Sci. 98, 2549–2572 (2009)
C. Keck, B. Muller, Eur. J. Pharm. Biopharm. 62, 3–16 (2006)
C.A. Lipinski, J. Pharmacol. Toxicol. Methods. 44, 235–249 (2000)
C. A Lipinski, in Pharmaceutical Profiling in Drug Discovery for Lead Selection, ed. by R.T. Borchardt, E.H. Kerns, C.A. Lipinski, D.R. Thakker, B. Wang (American Association of Pharmaceutical Scientists, 2004), pp. 93–125
S. Stegemann, F. Leveiller, D. Franchi, H. de Jong, H. Lindén, Eur. J. Pharm. Sci. 31, 249–261 (2007)
B.C. Hancock, M. Parks, Pharm. Res. 17, 397–404 (2000)
R. Meesata, H. Belmouaddine, J.-F. Allarda, C. Tanguay-Renaud, R. Lemay, T. Brastaviceanu, L. Tremblay, B. Paquette, J.R. Wagner, J.-P. Jay-Gerin, M. Lepage, M.A. Huels, D Houde, PNAS E2508–E2513 (2012)
Acknowledgments
The authors acknowledge J.-C. Leroux and J. Leblond for helpful discussions and access to experimental equipment, E. Nadezhina for the elemental analyses and Y. Drolet and J.-M. Rabanel for technical assistance. Financial support was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research (CIHR), the Groupe de Recherche Universitaire sur le Médicament (GRUM), the Fonds de la recherche en santé du Quebec (FRSQ) and the Canadian Foundation for Innovation (CFI).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, W., Sylvestre, JP., Bouvier, E. et al. Ultrafast laser processing of drug particles in water for pharmaceutical discovery. Appl. Phys. A 114, 267–276 (2014). https://doi.org/10.1007/s00339-013-8089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-8089-1