Abstract
Zooplanktivory is an important source of nutrients in corals, providing up to 35% of daily metabolic energy requirements in some species. However, little is known about coral zooplanktivory shortly after larval settlement and metamorphosis. In most species it is unclear if, when and under which conditions newly settled polyps are able to capture and ingest prey. This remains a critical knowledge gap, as zooplanktivory could allow coral settlers to replenish energy reserves shortly after metamorphosis, possibly improving settler condition during one of their most vulnerable life stages. Here, we documented the onset of prey (Artemia salina nauplii) capture in ten Caribbean coral species and assessed optimal water flow rates (WFR) for prey capture in five of these species. All species initiated zooplanktivory within six days following metamorphosis, with the exception of Acropora palmata which was never observed capturing nauplii during our 20-day study. Optimal WFR for prey capture varied among species, with Favia fragum displaying maximum prey capture rates in zero flow and Diploria labyrinthiformis most effectively capturing nauplii under WFR of 5–20 cm s−1. Under each species’ optimum WFR, prey capture abilities varied considerably, with F. fragum capturing up to one nauplius every two minutes compared to one nauplius every nine minutes in Colpophyllia natans. Using these findings, we make species-specific recommendations to optimize coral husbandry and larval-based restoration practices for these ten coral species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical scleractinian corals live in oligotrophic waters where food is scarce. As a result, they have evolved to be polytrophic, capable of exploiting both photoautotrophy and heterotrophy. Corals can therefore function at various trophic levels in their ecosystem, acting as primary producers, primary consumers, or as secondary and tertiary consumers (Goreau et al. 1971; Muscatine and Porter 1977). Their trophic versatility lies in their association with endosymbiotic Symbiodiniaceae dinoflagellates, which translocate ~ 95% of photosynthates (e.g., glycerol, amino acids, peptides, sugars) they produce to their coral host (Muscatine 1990). Photoautotrophy therefore is an important component of a coral’s energy budget and can provide well above 100% of a colony’s daily metabolic energy (DME) requirements for respiration and growth (Muscatine 1990; Grottoli et al. 2006). However, heterotrophy via zooplankton capture and uptake of particulate and dissolved matter can also constitute a large component of a coral’s diet, meeting 15% to 35% of DME requirements in healthy corals, and in some species supplying up to 66% of fixed carbon required for skeleton production (Houlbrèque and Ferrier-Pagès 2009). The relative contribution of heterotrophy to a coral’s energy budget can further increase under certain local environmental conditions (Palardy et al. 2005). For example, during bleaching events caused by thermal anomalies, Montipora capitata colonies lacking symbionts are nonetheless capable of acquiring over 100% of their DME requirements from heterotrophic feeding alone (Grottoli et al. 2006), illustrating the importance of heterotrophy as a complementary or alternative source of energy in some coral species.
While the role of heterotrophy is quite well-documented in adult corals, much less is known about heterotrophy during their earliest life stages. This is despite the fact that heterotrophy could be an important driver of survival and growth in young corals, especially in species for which symbiont transmission occurs horizontally (Suzuki et al. 2013). These species acquire their algal endosymbionts from the environment, either during the pre- or post-settlement phase (Aihara et al. 2019), rather than vertically from their parent during internal development (Hirose et al. 2001). Thus, unless capable of heterotrophy, they start their benthic life dependent upon lipid reserves provided by their maternal colony (lecithotrophy) (Graham et al. 2008). These lipid reserves are rapidly depleted during a larva’s pelagic life and its transition to a benthic stage, with active swimming and metamorphosis both incurring great energetic costs (Edmunds et al. 2013). Replenishing energy reserves shortly after metamorphosis can therefore be crucial to the health and growth of young settlers (Lewis 1974; Petersen et al. 2008). Yet, in most coral species, it remains unclear if, when and under which conditions newly settled corals are able to acquire nutrients via zooplanktivory.
Most adult corals are efficient meso-zooplankton (0.2–20 mm) predators (Houlbrèque and Ferrier-Pagès 2009). To capture prey, they can use tentacles armed with nematocysts that are discharged upon contact with a prey and/or entanglement by mucus nets or filaments (Lewis and Price 1975; Huettel et al. 2006). Following capture, prey items are moved into the polyp mouth by tentacles or deposited on the oral disk where they are immobilized in mucus and drawn into the mouth by ectodermal cilia. In young coral settlers, feeding structures (i.e., mouth and tentacles) develop within a day or two after metamorphosis (AM) (Randall et al. 2020). It is, however, unclear when they become functional and enable prey capture and ingestion. To date, a handful of studies documenting the onset of zooplanktivory in coral settlers exist and exclusively concern Pacific species. For example, Pocillopora damicornis and Acropora hyacinthus can already actively feed on Artemia nauplii (hereafter nauplii) starting two days AM (Toh et al. 2013a, 2013b), whereas Seriatopora caliendrum was only observed capturing prey starting five to six days AM (Cumbo et al. 2012). Such differences among species may have important implications for physiological and metabolic processes in corals during one of their most vulnerable life stages, but remain poorly studied especially in Caribbean species.
Water flow is a key factor determining plankton capture rates by reef organisms (Sebens et al. 1998; Wijgerde et al. 2012). Waves and currents act as a plankton pump along the benthos, providing suspension feeders such as corals with a constant input of zooplankton including copepods, crab zoeae and fish larvae (Leichter et al. 1998). The speed at which these organisms are transported by water on a reef influences a coral’s capacity to capture and feed on these particles (Leichter et al. 1998; Sebens et al. 1998). At very low water flow rates (WFR) (< 5 cm s−1), prey supply is limited, and prey can easily evade a coral’s grip by swimming away against weak currents. At moderate WFR (5–15 cm s−1) prey supply increases, and many prey are no longer capable of escaping coral tentacles (Sebens et al. 1996a; Wijgerde et al. 2012). At very high WFR (> 30 cm s−1), delivery of prey is high but the coral’s capacity to capture them is restrained by elevated kinetic energy levels that prevent prey from adhering to tentacles (Sebens and Johnson 1991). At such high WFR, polyp deformation can also reduce the surface area available for prey capture (Sebens 1997). Under these conditions, polyps may even completely retract their tentacles and stop feeding (Dai and Lin 1993). However, coral species are not equally affected by water flow. Distinct water circulation dynamics around corals with contrasting sizes and growth forms can influence their ability to capture prey. For example, in colonial corals exposed to high WFR, upstream polyps create small eddies that reduce flow around downstream polyps. The latter as a consequence benefit from increased prey capture rates, a strategy which solitary corals cannot rely on. As such, optimal WFR in colonial corals are up to 10 cm s−1, but below 1.25 cm s−1 in single-polyp corals (Wijgerde et al. 2012). Despite the key role of water flow in modulating prey capture rates in corals, it remains unclear how it might influence newly settled polyps during a feeding effort, especially given their small size (< 1 mm in diameter), solitary bauplan and lack of a well-developed skeleton which may render them more vulnerable to polyp deformation and prey evasion.
Studying the feeding habits of newly settled corals would not only improve our understanding of their capacity to obtain nutrients in their natural habitat, but also help increase the sustainability and (cost)effectiveness of larval-based coral husbandry and restoration. Feeding is an important component of coral husbandry, providing captive corals with key nutrients that benefit their overall health (Houlbrèque and Ferrier-Pagès 2009; Osinga et al. 2011; Leal et al. 2014). When propagating coral settlers with the goal of restoring wild populations, supplying them with appropriate and timely nutrient sources prior to outplanting may also improve their growth and survival on the reef (Latijnhouwers et al. 2022). Nonetheless, while suitable feeding regimes have been tailored for many adult coral species (e.g., Conlan et al. 2019; Wijgerde et al. 2012), feeding protocols are currently lagging for young corals, particularly for newly settled polyps.
In this study, we documented the onset of zooplanktivory in ten Caribbean coral species with a wide range of life histories, including eight broadcast spawning species with aposymbiotic settlers and two brooding species with symbiotic settlers. We provided them with brine shrimp nauplii (Artemia salina) starting immediately after metamorphosis and monitored the daily proportion of settlers first able to capture these prey. For five of these species, we also determined optimal WFR for maximal prey capture rates by exposing single polyp settlers to a range of WFR naturally occurring on reefs (0 to 35 cm s−1) (Sebens and Johnson 1991) inside a respirometric flow chamber. We use our findings to make species-specific recommendations to optimize practices for feeding of these coral species during their earliest and most vulnerable life stages.
Materials and methods
Study species
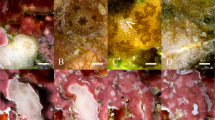
Feeding assays were performed between August 2018 and October 2020 with settlers of ten Caribbean corals species, including eight broadcast spawning species (Acropora palmata, Orbicella (formerly Montastraea) faveolata, Diploria labyrinthiformis, Colpophyllia natans, Pseudodiploria strigosa, Montastraea cavernosa, Dichocoenia stokesii and Siderastrea siderea) and two brooding species (Favia fragum and Porites porites) (Fig. 1). Together these species represent a broad range of life histories including different growth forms, reproductive strategies and algal symbiont transfer modes (Supplementary Table S1). A. palmata typically grows in shallow (0–5 m) and highly hydrodynamic habitats, whereas all other species can be found in a broader depth range and ecological conditions (Bak 1975). All experiments were conducted at the CARMABI Marine Research Station on the Caribbean island of Curaçao (12.1°, − 68.6°), with the exception of F. fragum for which adult colonies were exported from Curaçao to Wageningen University Research (WUR) in The Netherlands (CITES Permit NO: 18CW002) where larval release and feeding experiments took place.
The ten Caribbean coral species included in this study, at the adult stage and as recently settled polyps. Scale bar is 500 µm for each settler photo. Photo credits: adult colony of A. palmata by Selvaggio P., O. faveolata by Vermeij MJA, M. cavernosa, S. siderea, D. stokesii, P. strigosa, C. natans, D. labyrinthiformis, F. fragum by ter Horst L, and P. porites by Chamberland VF, primary polyps of all species by Chamberland VF except C. natans and D. stokesii by Geertsma RC
Gamete collections, fertilization, and larval rearing
Brooded larvae were obtained ex situ by harvesting adult colonies from the wild and monitoring daily larval release in an aquarium system as described in Chamberland et al. (2017). Adult F. fragum and P. porites colonies were sourced from the Curaçao Sea Aquarium reef (12.0834°, − 68.8954°) at a depth of approximately 4 m, after which they were transferred to a 50-L open circulation aquarium system at CARMABI. F. fragum colonies exported to WUR were kept in a 300-L closed circulation aquarium system. For details on this system and on transport of corals by air refer to supplementary information (SI). On expected planulation days (F. fragum: Szmant-Froelich et al. 1985, P. porites: Chamberland VF, unpub. data; Supplementary Table S1), colonies were individually placed in a 1-L beaker overnight in which constant water inflow was provided through plastic tubing. This caused the beaker to overflow and buoyant larvae were forced over the beaker’s handle into a semi-submerged collection cup equipped with a 100 µm nylon sieve at its bottom. Using plastic pipettes, larvae were then carefully transferred into clear plastic 550-mL containers filled with 500 mL of 0.5 µm-filtered seawater (FSW) and kept in the laboratory until experiments began.
Gametes from broadcast spawning species were harvested in situ from wild populations at the Water Factory (12.1091°, − 68.9544°) and Sea Aquarium reefs on predicted spawning nights (Vermeij et al. 2021, Supplementary Table S1). Gamete collections and fertilization were carried out following techniques previously developed by our team (e.g., Chamberland et al. 2015; Marhaver et al. 2015). Further details can be found as supplementary information (SI).
Embryos or larvae were distributed among multiple clear 2-L polystyrene containers (Dart Container Corporation, MI, USA) filled with 1.0 L of FSW at densities below one larva mL−1 (Vermeij et al. 2006). Maintenance of the culture consisted of removing dead larvae and biofilms from the water surface daily, and ~ 50% water changes in each container every other day. After larvae became motile and started swimming downwards in search of settlement surfaces, they were assigned to the two experiments described below. Water temperature was kept at 28 °C throughout the experiments, corresponding to daily average seawater temperature between August and November 2018 and 2020 (NOAA Coral Reef Watch 2018, 2020), and settlers were reared under ~ 12:12 h light:dark daily light cycles.
Artemia nauplii culture
In both experiments described below, recently hatched (< 12 h) brine shrimp (Artemia salina) nauplii were used as a source of food to study zooplanktivory in young corals in function of age and WFR. While natural zooplankton sources on a coral reef constitute of a variety of species of different size classes and nutrient contents (Carillo-Baltodano and Morales-Ramirez 2016), we chose to perform this study exclusively with brine shrimp nauplii given that they (i) are analogous to zooplankton occurring on reefs such as crab zoeae, which are a natural food source for corals (Sebens et al. 1996a), (ii) adult and juvenile corals as well as primary polyps of multiple species are known to feed on A. salina nauplii in ex situ cultures (Lewis and Price 1975; Toh et al. 2013a, 2013b; Cumbo et al. 2012, Petersen et al. 2008) and (iii) are large enough (400–600 µm in diameter) to easily confirm prey capture and ingestion with the naked eye.
Batches of A. salina cysts (Great Salt Lake Artemia cysts, Artemia International LLC, Fairview, OR, USA) were hatched daily by incubating them for 24 h in 1.2 L of FSW at 30 ppt and 28 °C, inside a 1.5-L plastic cone with aeration provided by an air pump (Whisper Tetra, Blacksburg, VA, USA), as indicated by the manufacturer. After hatching, the A. salina nauplii (hereafter nauplii) were rinsed with FSW over a 100 µm cell strainer (Stellar Scientific Swish, Baltimore, MD, USA) to prevent particle and nutrient contamination, and resuspended in 35 ppt FSW to reestablish ambient seawater salinity prior to feeding. A prey concentration of 3600 L−1 nauplii was used in all feeding trials. This concentration was achieved by homogenizing the stock solution described above, counting and averaging the number of nauplii in six 1 mL samples, and then adding the required volume of stock solution to reach 3600 nauplii L−1 in the feeding assays. This concentration is two to three orders of magnitude higher than natural zooplankton concentrations on Caribbean reefs reported by Jacobson & Edmunds (2010). Such elevated concentrations are typically used for feeding experiments using nauplii (Sebens et al. 1996a; Petersen et al. 2008; Toh et al. 2013b) to prevent prey depletion during the incubation period, or low prey concentrations resulting in no or low numbers of capture events (Sebens et al. 1996a).
Experiment 1: onset of prey capture
To track the onset of prey capture in recently settled coral polyps, larvae of each of the ten study species were first distributed among multiple standard Petri dishes (9 cm diameter, 1.5 cm height). Each dish was filled with 10 mL of FSW, after which a thin layer of crushed crustose coralline algae (CCA, Hydrolithon boergesenii) was added to promote settlement (Ritson-Williams et al. 2016). CCA was ground into fine dust (< 1 mm diameter) so that larvae would not completely attach to loose CCA particles, and at least partially adhere to the dish. Dishes were inspected twice daily so that the location and timing of metamorphosis could be recorded. For all species, metamorphosis occurred within a day after adding CCA after which settlement was stopped. All remaining planktonic larvae were removed from the dish, water was replaced with fresh FSW and CCA residues were discarded to maintain water quality. This resulted in dishes containing multiple newly settled larvae of the same age that could be used for feeding assays starting on the same day as metamorphosis (day 0 AM) (Supplementary Table S2). The number of experimental dishes and number of settlers per dish varied per species depending on larval availability and propensity to settle, and ranged from 1 to 34 dishes, each with 1 to 12 settlers (Supplementary Table S2). Before starting with feeding trials, each settler was mapped and received a unique number that enabled tracking of age (number of days AM) and onset of prey capture.
For the feeding assays, settlers were provided with ad libitum nauplii for 20 min daily for P. strigosa, P. porites and S. siderea, and for 60 min daily for all other species (Supplementary Table S2). The shorter incubation period for the former species was due to time constraints when rearing all three species simultaneously in October 2020 with limited manpower. Nonetheless, a 20 min feeding effort is thought to be sufficient to observe zooplanktivory in corals (Sebens et al. 1996a). These feeding assays were always performed in the afternoon between 12:00 and 19:00. The prey capture ability of each settler was recorded under a stereomicroscope (Amscope, CA, USA). After each trial, water in the dishes was replaced with fresh FSW to remove any non-captured nauplii and waste products from the feeding process (e.g., mucus, nutrients). In all species, prey capture was monitored daily for ten days AM or until at least 70% of settlers had been observed capturing nauplii at least once. For A. palmata, C. natans and D. labyrinthiformis, prey capture rates were monitored for a longer period of 20 days AM, with daily monitoring on days 0–10 AM and every other day onwards (Supplementary Table S2). In order to confirm if captured nauplii were fully ingested, at least one settler per species was monitored until complete ingestion following a prey capture event.
Experiment 2: influence of water flow rate on prey capture rates
Square PVC-tiles (50 × 50 × 3 mm, L × W × H) were used as settlement surfaces. These tiles were designed to fit exactly into the tile holder of a respirometric flow chamber in which the influence of WFR on prey capture rates was tested (Fig. 2). For broadcast spawning species, PVC-tiles were placed in 30-L plastic containers (36 × 31 × 24 cm, L × H × W; Sterilite, MA, USA) filled with 25 L of FSW. Depending on the available number of larvae, up to 25 tiles and 4,000 to 6,000 conspecific larvae were added in each container, and multiple containers were used if necessary. For F. fragum, 15 larvae with a single PVC-tile were added to 550-mL plastic containers. Similar to Experiment 1, finely crushed CCA particles were scattered on the surface of the tiles to induce settlement. Once at least 20 broadcast-spawned larvae, or one brooded larva had settled and metamorphosed on a tile, CCA residues were removed from the tile by slowly agitating and rinsing it with FSW using a glass pipette. Tiles with settlers were then transferred into another clean 30-L container in 20 L of FSW until settlers were capable of capturing nauplii based on results obtained in Experiment 1. To maintain water quality, water movement and aeration inside these containers was created by two airlifts placed at opposite corners of the containers and connected to an aquarium air pump (Whisper Tetra, Melle, Germany), and water was fully exchanged every other day. Due to logistical constraints as well as differences in larval availability and settlement rates among species, the total number of tiles and settlers that could be used for prey capture rate assays in the flow chamber was not equal across species (Supplementary Table S2) and P. strigosa, D. stokesii, S. siderea and P. porites could not be included in this experiment.
Respirometric flow chamber (version III). a Picture of the top/side of the flow chamber b Top-view schematic representation of the flow chamber with location of each part of the chamber. Figure adapted from Schutter et al. (2011)
Tiles harboring settlers were placed in a respirometric flow chamber (WUR, The Netherlands) (Fig. 2a). Following Wijgerde et al. (2012), water flow was created using a modified paddle wheel powered by a direct current (DC) motor with a three-channel incremental encoder and a line driver that allows for precise control of the rotational speed (Maxon Motor Benelux B.V., Enschede, The Netherlands). An electronic positioning user interface software (EPOS Version 3.0, Maxon Motor Benelux B.V., Enschede, The Netherlands) generated a water flow across the tile holder that could range in speed from 0 to 35 cm s−1. This range was set according to calibration tables used by Schutter et al. (2011) and corresponded to naturally occurring WFR on coral reefs (Sebens and Johnson 1991).
First, the maximum WFR (maxWFR) each species could withstand was determined based on the average WFR that would result in polyp deformation (n = 3 settlers per species). This was achieved by placing individual PVC-tiles with settlers in the chamber, and after 15 min acclimation, the WFR was increased gradually, by 1 cm s−1 every minute, until polyp deformation was observed in the upstream settler. The PVC-tile was then removed, and the trial was repeated with two more settlers. In this context, polyp deformation was characterized by the flattening of tentacles against the polyp’s corallum, therefore indicating that a polyp is no longer capable of capturing, retaining and ingesting prey (Sebens 1997). Species-specific maxWFR were used as maximum water flow boundaries in the respirometric flow chamber. Due to technical difficulties with the flow chamber motor, no maxWFR was determined for F. fragum and the maxWFR tested was of 19 cm s−1.
Each species’ optimal WFR (optWFR) for prey capture was then assessed at intervals of 5 cm s−1, starting at 0 cm s−1 until reaching this species’ maxWFR. Every trial included one settler on one tile, i.e. each settler that was monitored originated from a different tile given that other settlers on that same tile are unavoidably fed during a trial. The number of trials per water flow rate varied among species and ranged between 3 and 10 depending on the number of available settlers (Supplementary Table S2). Using the rotating coral tile holder (Fig. 2b), each monitored settler was rotated upstream of other polyps to prevent (1) alteration of water flow dynamics by other settlers, (2) downstream prey depletion possibly resulting in lower prey capture rates and (3) feeding on mucus possibly secreted by upstream polyps (Wijgerde et al. 2012). Prey capture was defined as a nauplius observed adhering to the surface of a coral settler for at least ten seconds (Wijgerde et al. 2011). Each tile was placed in the flow chamber for 15 min before nauplii were added to allow acclimation before a trial started. During this acclimation phase, the settler was monitored with a handy cam (HDRCX505VE, Sony Corporation, Tokyo, Japan). Given the large number of assays per species and WFR (up to 14) and their duration (up to one hour), it was impossible to run all experiments in the respirometric chamber on the same day and at the same time of the day. It is therefore possible that both the coral’s circadian rhythm (Sorek et al 2014) and the crepuscular zooplankton migration on reefs (Yahel et al. 2005) could have influenced the settlers’ feeding propensity. Thus, in an effort to ensure that all settlers were active despite being monitored at different times, we initiated the assays only if they displayed extended tentacles. In addition, different WFR treatments were randomized for each species, such that potential effects of time of day on polyp feeding activity were spread across treatments.
Once ready to initiate an assay, WFR was increased by 1–2 cm s−1 every minute during the acclimation phase until the target WFR was achieved. Feeding trials were performed under a dim light (8 µmol s−1 m−2) provided by a LED ring lamp. This prevented nauplii phototaxis towards or away from coral settlers. Once nauplii were added, the settler was filmed for 30 min at 1440 × 1080 pixels and 25 fps. The tile was then removed and placed in a container with clean FSW to prevent transfer of nauplii to other unfed settlers. The chamber was drained, flushed with freshwater, then with FSW and completely refilled before the next trial. The recording was then viewed to quantify prey capture rates for each settler (nauplii hr−1). A species’ optWFR was defined as the WFR or range of WFR at which it captured the highest number of nauplii per hour. In addition to recording prey capture rates, the production of mucus and the formation of nauplii aggregations near a settler were noted. Mucus production, along with physical trapping by tentacles and nematocysts can constitute a prey trapping mechanism, and nauplii aggregations in the vicinity of a coral settler may indicate that extracoelenteric digestion may be occurring.
Data analysis
In Experiment 1, the onset of prey capture data was analyzed using descriptive statistics. Due to time constraints and a limited amount of larvae available for P. strigosa, P. porites and S. siderea, multiple settlers were tracked in a single dish, therefore lacking true replication. For all species, we therefore expressed values as cumulative percentages of settlers that captured prey over time across all dishes. In Experiment 2, prey capture rates were assessed under a series of (discrete) water flow rates and were non-normally distributed (Kolmogorov–Smirnov, p < 0.05). Nonparametric Kruskal–Wallis H-tests were therefore performed to determine whether WFR influenced prey capture rates. We then ran Mann–Whitney post hoc U tests as pairwise comparisons to determine optimum WFR for prey capture when applicable. Mann–Whitney U tests were also used when comparing two species’ maximum prey capture rates. A Bonferroni correction for multiple hypothesis testing was applied. All tests were performed with SPSS 24.0.0 (IBM Corp. 2016).
Results
Experiment 1: onset of prey capture
All coral species with the exception of A. palmata were observed capturing nauplii during this study. In these species, prey capture first occurred between day 1 and 6 AM, and by day 8 AM, 70% to 100% of all live settlers had been observed capturing nauplii at least once (Fig. 3). The latest onset of zooplanktivory was observed in O. faveolata, with only 50% (n = 10) of settlers initiating feeding by day 7 AM, whereas the earliest onset of zooplanktivory occurred in F. fragum with 3% (n = 34) of settlers observed capturing prey on day 1 AM and more than 50% of settlers by day 3 AM (Fig. 3). Video-monitoring confirmed that nauplii capture was followed by ingestion of prey (Supplementary Video 1 and 2 showing F. fragum and D. stokesii, respectively) in all species.
Onset of prey capture in newly settled polyps of 10 Caribbean coral species. Gray bars indicate the proportion of settlers observed capturing nauplii for the first time, black bars are the cumulative proportion of settlers capturing nauplii, and hatched bars indicate the first day on which at least 50% of settlers were observed capturing nauplii. n indicates the number of settlers for which prey capture was monitored during the study period. No prey capture was observed in A. palmata over 20 days. The number of experimental dishes and number of settlers per dish varied per species depending on larval availability and propensity to settle, and ranged from 1 to 34 dishes, each with 1 to 12 settlers (Supplementary Table S2)
Experiment 2: influence of water flow on prey capture rates
A. palmata was excluded from Experiment 2, because it was never observed feeding in Experiment 1 (Fig. 3). Settlers of all five species displayed extended tentacles following the 15 min acclimation period in the respirometric flow chamber and were not observed excreting visible mucus strands. Aggregations of nauplii in the vicinity of settlers were also never observed. As in Experiment 1, nauplii capture followed by complete ingestion was recorded for all species.
Polyp deformation occurred at different WFR for different species (25 cm s−1: C. natans and O. faveolata, 30 cm s−1: M. cavernosa, 35 cm s−1: D. labyrinthiformis) (Fig. 4), representing each species’ maxWFR under which prey capture rates were tested. WFR had a significant influence on prey capture rates in all five species (Kruskal–Wallis H-test, Supplementary Table S3) Fig. 3). For D. labyrinthiformis, optimal water flow rates (optWFR) for prey capture ranged between 5–20 cm s−1. This species captured 8 to 20 times more nauplii at 15 cm s−1 than at either zero or very high water flow (25–30 cm s−1) (Mann–Whitney U test, Supplementary Table S3) (Fig. 4). In contrast, F. fragum settlers were most effective in capturing nauplii without water flow, capturing almost 40 times more nauplii h−1 at 0 cm s−1 than at 19 cm s−1 (Mann–Whitney U test, Supplementary Table S3) (Fig. 4). In C. natans, optWFR ranged from 5 to 10 cm s−1. When exposed to a WFR of 10 cm s−1, this species captured 3 times more nauplii than at 0 cm s−1 and 6 to 17 times more nauplii than at 15 cm s−1 or higher (Mann–Whitney U test, Supplementary Table S3) (Fig. 4). M. cavernosa settlers captured nauplii under WFR ranging from 0 to 20 cm s−1, and were unable to feed at 25 cm s−1. As for O. faveolata, settlers exhibited optimal prey capture rates at WFR between 0 and 10 cm s−1, with capture rates declining at higher WFR (Fig. 4). This species captured six times more nauplii at 5 cm s−1 than at 15 cm s−1 (Mann–Whitney U test, Supplementary Table S3) (Fig. 4).
Prey capture rates of settlers of the five study species as a function of water flow rate. Each bar represents the mean number of nauplii captured by individual settlers under each water flow rate tested. Letters above bars indicate significantly different groupings. The number of trials per water flow rate varied among species and ranged between 3 and 10 depending on the number of available settlers (Supplementary Table S2)
When settlers were exposed to their optWFR, F. fragum was able to capture the highest number of nauplii per hour (30.3 ± 6.3 nauplii h−1) of all species, three to four times more than that of D. labyrinthiformis (10.0 ± 2.0 nauplii h−1), O. faveolata (7.6 ± 1.7 nauplii h−1), and C. natans (7.0 ± 2.2 nauplii h−1) (Supplementary Table S4) (Fig. 5).
Discussion
This is the first study documenting aspects of heterotrophy in newly settled polyps of Caribbean corals. We tracked the onset of zooplanktivory in settlers of eight broadcast spawning species and two brooding species (Fig. 1), and further quantified the influence of water flow on the prey capture ability in settlers of five of these species. Our results (summarized in Table 1) highlight species-specific differences in feeding behavior which may have significant implications for physiological and metabolic processes in corals shortly after metamorphosis, as well as useful applications to optimize recruit husbandry practices.
Early onset of zooplanktivory
All species, with the exception of A. palmata, exhibited an early onset of zooplanktivory with first nauplii captures observed between one and six days following metamorphosis (Fig. 3). These results are comparable with the three Indo-Pacific species S. caliendrum, P. damicornis and A. hyacinthus, all initiating prey capture between two and six days AM (Cumbo et al. 2012; Toh et al. 2013a, 2013b). Together, these observations suggest an important contribution of heterotrophy to the DME requirements of young corals shortly after transiting to their benthic stage. In adult corals, heterotrophy increases protein and lipid availability for tissue and membrane constituents, as well as essential amino acids involved in skeletogenesis (reviewed in Houlbrèque and Ferrier-Pagès 2009). Heterotrophy is therefore likely to be a critical source of nutrients in young settlers as well. However, because ex situ feeding without (confirmed) capture can lead to nutrient buildup, enhanced algae growth and, as a result, impaired coral growth (Forsman et al. 2012), current larval-based coral husbandry and restoration practices often provide settlers with live feeds (days to weeks) later in life (Petersen et al. 2008; Toh et al. 2014, but see Lewis 1974), possibly withholding beneficial nutrient sources earlier on. Corals follow a type III survivorship curve, and typically suffer high mortality rates in the first weeks following metamorphosis (Vermeij and Sandin 2008). In settlers reared in filtered seawater (without plankton) and not provided with additional food sources, starvation could be a possible cause of death, especially in species not yet hosting their obligate algal symbionts. In a few species, providing settlers with live feeds early on (starting ≤ 2 weeks AM) was shown to drastically increase growth rates, 3.5- to fivefold in F. fragum (11-month feeding period: Lewis 1974; 5-month feeding period: Petersen et al. 2008), tenfold in P. damicornis (6-month feeding period: Toh et al. 2014) and 1.2-fold in D. labyrinthiformis (1-week feeding period: Latijnhouwers et al. 2022). Fed and larger P. damicornis and D. labyrinthiformis settlers were in turn almost twice as likely to survive past three months following outplanting to the wild, relative to unfed conspecifics (Toh et al. 2014; Latijnhouwers et al. 2022). Combined with our results, these studies demonstrate that providing settlers with live zooplankton feeds soon after metamorphosis may help reduce early life bottlenecks in some coral species. It is important to note that in this study and others (Cumbo et al. 2012; Toh et al. 2013a, 2013b), the onset of coral zooplanktivory was investigated in the absences of water flow and exclusively with Artemia nauplii as food source. Given that it is unknown if and how water flow influences ontogeny, and if settlers would preferentially and more rapidly feed on other food types, further investigations are warranted to include a larger spectrum of diets in a variety of ex situ and in situ settings, and for a broad range of coral species.
We observed important differences in the onset of prey capture among species (though we recommend repeating this experiment with proper replication for P. strigosa, S. siderea and P. porites). Over half of F. fragum and P. porites settlers were able to feed on live prey two to three days AM, whereas in O. faveolata, prey capture surpassed 50% of settlers only seven days AM (Fig. 3). The onset of zooplanktivory seemingly occurs even later in A. palmata settlers which were never observed capturing prey in our 20-day observation period (Fig. 3) despite their larger size and more developed tentacles relative to other broadcast spawners (Fig. 1). Such delayed onsets of prey capture could have important consequences especially for aposymbiotic settlers. During that time, unless capable of assimilating dissolved organic nutrients or ingesting plankton items other than Artemia nauplii, aposymbiotic settlers are unable to renew energy reserves that may be depleted after larval settlement and metamorphosis. In contrast, F. fragum and P. porites settlers may be at a large advantage given that they already host Symbiodiniacea (Szmant-Froelich et al. 1985; Tomascik and Sander 1987) (Fig. 1) and can capture prey early (one to three days AM, Table 1) (Fig. 2), thus benefiting from both autotrophic and heterotrophic energy sources shortly after metamorphosis.
It remains unclear how to attribute these differences in feeding behavior among species. Polyp diameter was hypothesized to be a good indicator of prey capture ability in adult corals in the mid 70’s (Porter 1976), although this was later disproved (Palardy et al. 2005, 2006). The similar assumption that larger settlers are able to feed on zooplankton earlier following metamorphosis is also not supported by our observations, with relatively small P. porites settlers (~ 800 µm diameter; ter Horst L, unpub. data) initiating feeding one day earlier than as F. fragum (Fig. 3), the species with the largest settlers (~ 1500 µm diameter; Geertsma RC, unpub. data) studied here (Table 1). Furthermore, A. palmata produces the largest settlers (~ 1080 µm diameter; Mendoza Quiroz S, unpub. data) of all broadcast spawning species included in this study (Table 1), but did not initiate zooplanktivory (on nauplii) within our 20-day study period (Fig. 3), whereas all other broadcast spawning species with smaller primary polyps (485–700 µm diameter; Chamberland VF, unpub. data) were able to capture prey within six days AM (Table 1) (Fig. 3). Thus, the onset of prey capture in coral settlers does not appear to be a function of polyp size, and could instead be linked to tentacle length. Here, although we did confirm tentacle extension prior to the start of experiments, we did not measure tentacle length. Different onsets of prey capture among species could also be linked to the timing of development of feeding structures (i.e., mouth and tentacles) and/or nematocyst synthesis, composition, sensitivity, and distribution in tissues, which are poorly described in corals. In other Cnidaria (Cubozoa and Scyphozoa), changes in gross morphology and in cnidome composition and distribution during development coincide with shifts in diet, from smaller invertebrates to larger vertebrates (Chironex fleckeri; McClounan and Seymour 2012) and in site of prey capture, from manubrium to tentacles (Cyanea capillata; Higgins et al. 2008). Although the presence of nematocysts was recently confirmed in larvae of O. faveolata, C. natans, P. strigosa, M. cavernosa, S. siderea (Chamberland VF, pers. obs.) and F. fragum (Geertsma RC, pers. obs.), their role during prey capture events shortly after metamorphosis is unknown.
It further could be expected that symbiotic settlers have a lower urgency to develop feeding structures and capture zooplankton compared to aposymbiotic settlers that cannot acquire energy via autotrophy, and may therefore exclusively rely on heterotrophy to meet their metabolic demands. This however does not appear to be the case, given that all four brooding species for which the onset of prey capture has yet been documented (P. porites, F. fragum: this study; S. caliendrum: Cumbo et al. 2012; P. damicornis: Toh et al. 2013b) exhibit an early onset of zooplanktivory (one to six days AM) (Table 1). Altogether, these findings suggest that early zooplanktivory is vital even in species with vertical symbiont transmission.
Delayed onset of zooplanktivory in Acropora palmata settlers
A. palmata is the only species not observed capturing prey in this study, despite the fact that adult colonies are known to prey on Artemia nauplii when provided with them in situ (Lewis and Price 1975). This observation differs from the Indo-Pacific A. hyacinthus which is capable of capturing prey as early as two days AM, highlighting that the mechanisms involved in early prey capture are species-specific and not conserved at the genus level. Important to note is that A. palmata is most commonly found in shallow (0–5 m) reef habitats exposed to high wave energy and high light levels (Bak 1975). This species may therefore (1) rely more heavily on autotrophy than heterotrophy given high light availability for photosynthesis at shallow depths, and as such may not initiate zooplanktivory until later in life, (2) produce nematocysts that are only triggered by prey items with higher kinetic energy levels and thus only feed in the presence of water flow (a condition not tested here), and/or (3) preferentially feed on other food types such as phytoplankton, as is the case for some octocorals species residing in strong water flow habitats (Fabricius et al. 1995). Thus, further research is warranted to identify other possible diets and water flow conditions that could promote zooplanktivory in A. palmata settlers. We also recommend facilitating early symbiont inoculation in A. palmata settlers to maximize early energy acquisition via photoautotrophy.
Species-specific influence of WFR on prey capture
To our knowledge, this is the first report of settler prey capture rates under different water flow rates. Water flow significantly impacted prey capture rates in settlers of all five study species. However, how it affected zooplanktivory varied among species, with species-specific maxWFR and optWFR. C. natans and O. faveolata were most prone to polyp deformation, which occurred at 20 cm s−1, compared to 25 and 35 cm s−1 in M. cavernosa and D. labyrinthiformis, respectively (Fig. 4). Past these values, prey capture and ingestion are very unlikely given that high WFR forces extended tentacles to flatten against the corallum or even cause tentacle retraction to avoid tissue damage (Dai and Lin 1993). In addition, prey traveling at high velocity are unlikely to adhere to tentacles after first contact (Sebens and Johnson 1991). Water flow near the benthos however rarely exceeds 5 cm s−1 (Hata et al. 2017). Polyp deformation is therefore unlikely to occur in the wild and should be avoided in a husbandry setting. Importantly, in this study we exposed coral settlers to unidirectional water flow, and did not study zooplanktivory under more complex water circulation patterns. Coral reefs comprise a diversity of habitats subjected to a diversity of water motion types, ranging from turbulent bi-directional, wave-induced flow in the shallows, to more stable unidirectional flow in deeper waters (Sebens and Johnson 1991). Fine-scale reef topographies further influence water flow, generating eddies and other turbulences that locally alter water flow velocity (Koehl et al. 2007), allowing coral larvae to settle in higher flow environments where they would otherwise be unable to do so (Hata et al. 2017). Similar processes may occur that allow settlers to feed on zooplankton even when exposed to sub-optimal WFR, whereby prey entering eddies and turbulences may reside longer in the vicinity of polyp tentacles and be more easily captured.
When considering WFR below 20 cm s−1, we observed significant and species-specific effects of water flow on zooplanktivory (Table 1). While higher unidirectional water flow rates enhanced prey capture abilities in some species, it reduced prey capture rates in others. For instance, D. labyrinthiformis and C. natans were twofold to tenfold more effective in capturing nauplii when WFR increased from 0 to 10–15 cm s−1, whereas F. fragum settlers were almost 40 times better at capturing prey without water flow than at 19 cm s−1 (Fig. 4). We further recorded remarkable differences in maximum prey capture rates under each species’ optWFR (Table 1), with F. fragum capturing one nauplius every two minutes compared to one nauplius every six to nine minutes in D. labyrinthiformis, O. faveolata and C. natans (Fig. 5). It remains unknown what factors underlie large differences in optWFR for prey capture and in maximum prey capture rates among species. It could be expected that larger settlers would exhibit higher prey capture rates. This study however did not include a sufficient number of species with different average polyp sizes to test this hypothesis. The five species studied under different WFR all produced fairly small settlers (~ 200–700 µm Φ, Table 1) with the exception of F. fragum (~ 1500 µm Φ, Table 1). In addition, large A. palmata settlers (1080 µm Φ, Table 1) did not capture any nauplii during the study period. This question may be better addressed by comparing maximum prey capture rates among conspecific settlers of different sizes. Lastly, it is also unclear if optWFR for prey capture coincide with water flow rates that are optimal for other key physiological processes including gas exchange within coral tissues, heat dissipation, sediment removal, growth and reproduction, or, alternatively, if WFR in a husbandry setting should be adjusted prior and after a feeding effort.
Despite the many unknowns that remain concerning the physiological and environmental processes influencing zooplanktivory in newly settled corals, this study provides useful data on species-specific feeding behavior for ten Caribbean species, including their onset of prey capture, optimal and maximum WFR for feeding, as well as maximum prey capture rates. This information will enable targeted feeding regimes for larval-based coral husbandry and restoration programs, ensuring that young coral settlers are provided with essential nutrients in a timely manner and under optimal water flow conditions for species with early onsets of zooplanktivory, while avoiding unnecessary food wastes and associated water quality declines for species with delayed onsets of zooplanktivory. Such ameliorations to current practices may in turn increase the sustainability and (cost)efficiency of coral larval propagation, whether for the aquarium trade or for reef restoration purposes.
References
Aihara Y, Maruyama S, Baird AH, Iguchi A, Takahashi S, Minagawa J (2019) Green fluorescence from cnidarian hosts attracts symbiotic algae. Proc Natl Acad Sci U S A 116:2118–2123
Bak RPM (1975) Ecological aspects of the distribution of reef corals inthe Netherlands Antilles. Bijdr Tot Dierkd 45:181–190
Carrillo-Baltodano A, Morales-Ramirez A (2016) Changes in abundance and composition of a Caribbean coral reef zooplankton community after 25 years. Rev Biol Trop 64(3):1029–1040
Chamberland VF, Vermeij MJA, Brittsan M, Carl M, Schick M, Snowden S, Schrier A, Petersen D (2015) Restoration of critically endangered elkhorn coral (Acropora palmata) populations using larvae reared from wild-caught gametes. Glob Ecol Conserv 4:526–537
Chamberland VF, Latijnhouwers KRW, Huisman J, Hartmann AC, Vermeij MJA (2017) Costs and benefits of maternally inherited algal symbionts in coral larvae. Proc R Soc London B Biol Sci 284:20170852
Conlan JA, Humphrey CA, Severati A, Parrish CC, Francis DS (2019) Elucidating an optimal diet for captive Acropora corals. Aquaculture 513:734420
Cumbo VR, Fan TY, Edmunds PJ (2012) Scleractinian corals capture zooplankton within days of settlement and metamorphosis. Coral Reefs 31:1155
Dai C-F, Lin M-C (1993) The effects of flow on feeding of three gorgonians from southern Taiwan. J Exp Mar Biol Ecol 173:57–69
Edmunds PJ, Cumbo VR, Fan TY (2013) Metabolic costs of larval settlement and metamorphosis in the coral Seriatopora caliendrum under ambient and elevated pCO2. J Exp Mar Biol Ecol 443:33–38
Fabricius KE, Genin A, Benayahu Y (1995) Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnol Oceanogr 40:1290–1301
Forsman ZH, Kimokeo BK, Bird CE, Hunter CL, Toonen RJ (2012) Coral farming: effects of light water motion and artificial foods. J Mar Biolog Assoc UK 92(4):721–729
Goreau TF, Goreau NI, Yonge CM (1971) Reef corals: autotrophs or heterotrophs? Biol Bull 141:247–260
Graham EM, Baird H, Connolly SR (2008) Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27:529–539
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Hata T, Madin JS, Cumbo VR, Denny M, Figueiredo J, Harii S, Thomas CJ, Baird AH (2017) Coral larvae are poor swimmers and require fine-scale reef structure to settle. Sci Rep 7:1–9
Higgins JE, Ford MD, Costello JH (2008) Transitions in morphology, nematocyst distribution, fluid motions, and prey capture during development of the scyphomedusa Cyanea capillata. Biol Bull 214:29–41
Hirose M, Kinzie RA III, Hidaka M (2001) Timing and process of entry of zooxanthellae into oocytes of hermatypic corals. Coral Reefs 20:273–280
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17
Huettel M, Wild C, Gonelli S (2006) Mucus trap in coral reefs: formation and temporal evolution of particle aggregates caused by coral mucus. Mar Ecol Prog Ser 307:69–84
IBM Corp. (2016) IBM SPSS Statistics for Windows, Version 24.0.
Jacobson LM, Edmunds PJ (2010) Long-term changes in the concentration of zooplankton and particulate material over a fringing reef in St. John, US Virgin Islands. Bull Mar Sci 86(3):763–772
Koehl AR, Strother JA, Reidenbach MA, Koseff JR, Hadfield MG (2007) Individual-based model of larval transport to coral reefs in turbulent, wave-driven flow: behavioral responsdes to dissolved settlement inducer. Mar Ecol Prog Ser 335:1–18
Latijnhouwers KRW, Ter Horst L, Schneider J, Van Duijnhoven J, Miller M, Vermeij MJAV, Chamberland, VF (2022) Feeding of coral settlers in large in situ mesocosms greatly increases their long term survival. Abstract Book, 15th International Coral Reef Symposium, p 980
Leal MC, Ferrier-Pagès C, Petersen D, Osinga R (2014) Coral aquaculture: applying scientific knowledge to ex situ production. Rev Aquac 8:136–153
Leichter JJ, Shellenbarger G, Genovese SJ, Wing SR (1998) Breaking internal waves on a Florida (USA) coral reef: A plankton pump at work? Mar Ecol Prog Ser 166:83–97
Lewis JB (1974) The importance of light and food upon the early growth of the reef coral Favia fragum (esper). J Exp Mar Biol Ecol 15:299–304
Lewis JB, Price WS (1975) Feeding mechanisms and feeding strategies of Atlantic reef corals. J Zool 176:527–544
Marhaver KL, Vermeij MJA, Medina MM (2015) Reproductive natural history and successful juvenile propagation of the threatened Caribbean Pillar Coral Dendrogyra cylindrus. BMC Ecol 15:9
McClounan S, Seymour J (2012) Venom and cnidome ontogeny of the cubomedusae Chironex fleckeri. Toxicon 60:1335–1341
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in coral reefs. In: Dubinsky Z (ed) Ecosystems of the World, Coral Reefs, vol 25. Elsevier Science Publishing Company, Inc, Amsterdam, The Netherlands, pp 75–87
Muscatine L, Porter JW (1977) Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27:454–460
NOAA Coral Reef Watch (2018) Daily global 5-km satellite virtual station time series data for Curaçao and Aruba, Jan. 01-Dec. 31, 2018. College Park, Maryland, USA. http://coralreefwatch.noaa.gov/satellite/vs/caribbean.php
NOAA Coral Reef Watch (2020) Daily global 5-km satellite virtual station time series data for Curaçao and Aruba, Jan. 01-Dec. 31, 2020. College Park, Maryland, USA. http://coralreefwatch.noaa.gov/satellite/vs/caribbean.php
Osinga R, Schutter M, Griffioen B, Wijffels RH, Verreth JAJ, Shafir S, Henard S, Taruffi M, Gili C, Lavorano S (2011) The biology and economics of coral growth. Mar Biotechnol 13:658–671
Palardy JE, Grottoli AG, Matthews KA (2005) Effects of upwelling, depth, morphology and polyp size on feeding in three species of Panamanian corals. Mar Ecol Prog Ser 300:79–89
Palardy JE, Grottoli AG, Matthews KA (2006) Effect of naturally changing zooplankton concentrations on feeding rates of two coral species in the Eastern Pacific. J Exp Mar Biol Ecol 331:99–107
Petersen D, Wietheger A, Laterveer M (2008) Influence of different food sources on the initial development of sexual recruits of reefbuilding corals in aquaculture. Aquaculture 277:174–178
Porter JW (1976) Autotrophy, heterotrophy, and resource partitioning in Caribbean reef-building corals. Am Nat 110:731–742
Randall CJ, Negri AP, Quigley KM, Foster T, Ricardo GF, Webster NS, Bay LK, Harrison PL, Babcock RC, Heyward AJ (2020) Sexual production of corals for reef restoration in the Anthropocene. Mar Ecol Prog Ser 635:203–232
Ritson-Williams R, Arnold SN, Paul VJ (2016) Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar Ecol Prog Ser 548:127–138
Schutter M, Kranenbarg S, Wijffels RH, Verreth J, Osinga R (2011) Modification of light utilization for skeletal growth by water flow in the scleractinian coral Galaxea fascicularis. Mar Biol 158:769–777
Sebens KP, Johnson AS (1991) Effects of water movement on prey capture and distribution of reef corals. Hydrobiologia 216–217:247–248
Sebens KP, Vandersall KS, Savina LA, Graham KR (1996a) Zooplankton capture by two scleractinian corals, Madracis mirabilis and Montastrea cavernosa, in a field enclosure. Mar Biol 127:303–331
Sebens KP, Grace SP, Helmuth B, Maney EJ, Miles JS (1998) Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastrea cavernoss and Porites porites in a field enclosure. Mar Biol 131:347–360
Sebens KP (1997) Adaptive responses to water flow: morphology, energetics and distribution of reef corals. In: Proceedings 8th international coral reef symposium, pp 1053–1058
Sorek M, Diaz-Almeyda EM, Medina M, Levy O (2014) Circadian clocks in symbiotic corals: the duet between Symbiodinium algae and their coral host. Mar Genomics 14:47–57
Suzuki G, Yamashita H, Kai S, Hayashibara T, Suzuki K, Iehisa Y, Okada W, Ando W, Komori T (2013) Early uptake of specific symbionts enhances the post-settlement survival of Acropora corals. Mar Ecol Prog Ser 494:149–158
Szmant-Froelich A, Reutter M, Riggs L (1985) Sexual reproduction of Favia fragum: lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull Mar Sci 37:880–892
Toh TC, Peh JWK, Chou LM (2013a) Early onset of zooplanktivory in equatorial reef coral recruits. Mar Biodivers 43:177–178
Toh TC, Peh JWK, Chou LM (2013b) Heterotrophy in recruits of the scleractinian coral Pocillopora damicornis. Mar Freshw Behav Physiol 46:313–320
Toh TC, Ng CSL, Peh JWK, Ben TK, Chou LM (2014) Augmenting the post-transplantation growth and survivorship of juvenile scleractinian corals via nutritional enhancement. PLoS ONE 9:e98529
Tomascik T, Sander F (1987) Effects of eutrophication on reef-building corals - III. Reproduction of the reef-building coral Porites porites. Mar Biol 94:77–94
Vermeij MJA, Sandin SA (2008) Density-dependent settlement and mortality structure the earliest life phases of a coral population. Ecology 89:1994–2004
Vermeij MJA, Fogarty ND, Miller MW (2006) Pelagic conditions affect larval behavior, survival, and settlement patterns in the Caribbean coral Montastraea faveolata. Mar Ecol Prog Ser 310:119–128
Vermeij MJ, Chamberland VF, Marhaver KL (2021) Coral Spawning Predictions for the Southern Caribbean 2007–2021. CARMABI, Curacao. https://www.researchstationcarmabi.org/predictions-for-coral-spawning-events-in-the-southern-caribbean-for-2022/
Wijgerde T, Diantari R, Lewaru MW, Verreth JAJ, Osinga R (2011) Extracoelenteric zooplankton feeding is a key mechanism of nutrient acquisition for the scleractinian coral Galaxea fascicularis. J Exp Biol 214:3351–3357
Wijgerde T, Spijkers P, Karruppannan E, Verreth JAJ, Osinga R (2012) Water flow affects zooplankton feeding by the scleractinian coral Galaxea fascicularis on a polyp and colony level. J Mar Biol 2012:1–7
Yahel R, Yahel G, Berman T, Jaffe JS, Genin A (2005) Diel pattern with abrupt crepuscular changes of zooplankton over a coral reef. Limnol Oceanogr 50(3):930–944
Acknowledgements
We extend our gratitude to Kristen Marhaver, Mark Vermeij, Maarten Hoftijzer, Ari Muskat, Lucas Tichy, and Daisy Flores for assistance with coral gamete collections. We thank Kristen Marhaver for providing the D. stokesii larvae used in our experiments, Tjitske Dalderup for assisting with experiments, Margaret Miller for providing valuable feedback on earlier versions of this manuscript, and Faisal Dilrosun for assisting with CITES export regulations. Lastly, we appreciate the fair and constructive criticism provided by Raphael Ritson-Williams and one anonymous reviewer, which helped improve an earlier version of this manuscript. This research received funding from the California Academy of Sciences.
Author information
Authors and Affiliations
Contributions
RCG and TW conceived the study, RCG and VFC designed the experiments, RCG, KRWL and VFC collected coral gametes and reared larvae for the experiments, RCG and KRWL carried out the experiments and collected the data, RCG and TW analyzed the data, RCG and VFC interpreted the data and wrote the manuscript. TW and KRWL reviewed earlier versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1 Dichocoenia stokesii settler capturing and ingesting a nauplii at the age of 4 days AM. The recording is sped up 100x right after prey capture, during ingestion. The scale bar is 500 μm (MP4 222616 kb)
Video 2 Favia fragum settler capturing and ingesting multiple nauplii at the age of 9 days AM. The entire recording is sped up 100x. The scale bar is 500 μm (MP4 295080 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geertsma, R.C., Wijgerde, T., Latijnhouwers, K.R.W. et al. Onset of zooplanktivory and optimal water flow rates for prey capture in newly settled polyps of ten Caribbean coral species. Coral Reefs 41, 1651–1664 (2022). https://doi.org/10.1007/s00338-022-02310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02310-2