Abstract
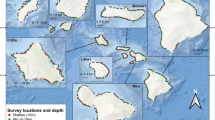
The impact of grazing by herbivorous fishes (Acanthuridae, Scaridae, and Pomacentridae) on low coral-cover reefs was assessed by measuring rates of benthic algal production and consumption on inshore and offshore reefs in the upper Florida Keys. Algal production rates, determined in situ with caged and uncaged experimental plates, were low (mean 1.05 g C m−2 day−1) and similar among reef types. Algal consumption rates were estimated using two different models, a detailed model incorporating fish bite rates and algal yield-per-bite for one species extrapolated to a guild-wide value, and a general regression relating fish biomass to algal consumption. Algal consumption differed among reef types: a majority of algal production was consumed on offshore reefs (55–100%), whereas consumption on inshore patch reefs was 31–51%. Spatial variation in algal consumption was driven by differences in herbivorous fish species composition, density, and size-structure among reef types. Algal consumption rates also varied temporally due to seasonal declines in bite rates and intermittent presence of large-bodied, vagile, schooling species. Spatial coherence of benthic community structure and temporal stability of algal turf over 3 years suggests that grazing intensity is currently sufficient to limit further spread of macroalgal cover on these low coral-cover reefs, but not to exclude it from the system.





Similar content being viewed by others
References
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ Biol Fish 28:189–214
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Birkeland C (1977) The importance of rate of biomass accumulation in early successional stages of benthic communities to survival of coral recruits. Proc 3rd Int Coral Reef Symp 1:15–21
Bohnsack JA, Harper DE (1988) Length–weight relationships of selected marine reef fishes from the southeastern United States and the Caribbean. NOAA Tech Mem NMFS-SEFC 215:1–31
Bohnsack JA, Harper DE, McClellan DB (1994) Fisheries trends from Monroe County, Florida. B Mar Sci 54:982–1018
Bruggemann JH, van Oppen MJH, Breeman AM (1994a) Foraging by the stoplight parrotfish Sparisoma viride. I. Food selection in different, socially determined habitats. Mar Ecol-Prog Ser 106:41–55
Bruggemann JH, Begeman J, Bosma EM, Verburg P, Breeman AM (1994b) Foraging by the stoplight parrotfish Sparisoma viride. II. Intake and assimilation of food, protein and energy. Mar Ecol-Prog Ser 106:57–71
Carpenter RC (1981) Grazing by Diadema antillarum (Philippi) and its effects on the benthic algal community. J Mar Res 39:749–765
Carpenter RC (1986) Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr 56:345–363
Carpenter RC (1988) Mass mortality of a Caribbean sea urchin: immediate effects on community metabolism and other herbivores. P Natl Acad Sci USA 85:511–514
Carpenter RC (1990) Mass mortality of Diadema antillarum II. Effects on population densities and grazing intensity of parrotfishes and surgeonfishes. Mar Biol 104:79–86
Chiappone M, Swanson DW, Miller SL, Smith SG (2002) Large-scale surveys on the Florida Reef Tract indicate poor recovery of the long-spined sea urchin Diadema antillarum. Coral Reefs 21:155–159
Choat JH (1983) Estimation of the abundances of herbivorous fishes and their grazing rates within reef systems. In: Proceedings of the inauguration Great Barrier Reef conference, pp 171–177
Choat JH, Bellwood DR (1985) Interactions amongst herbivorous fishes on a coral reef: influence of spatial variation. Mar Biol 89:221–234
Cowen RK, Agregian CR, Foster MS (1982) The maintenance of community structure in a central California giant kelp forest. J Exp Mar Biol Ecol 64:189–201
de Ruyter van Steveninck ED, Bak RPM (1986) Changes in abundance of coral reef bottom components related to mass mortality of the sea urchin Diadema antillarum. Mar Ecol-Prog Ser 34:87–94
Dustan P, Halas JC (1987) Changes in the reef-coral community of Carysfort Reef, Key Largo, Florida: 1974 to 1982. Coral Reefs 6:91–106
Ebeling AW, Laur DR, Rowley RJ (1985) Severe storm disturbances and reversal of community structure in a southern California kelp forest. Mar Biol 84:287–294
Edmunds PJ, Carpenter RC (2001) Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. P Natl Acad Sci USA 98:5067–5071
Ferreira CEL, Peret AC, Coutinho R (1998) Seasonal grazing rates and food processing by tropical herbivorous fishes. J Fish Biol 53:222–235
Foster SA (1987) The relative impacts of grazing by Caribbean coral reef fishes and Diadema: effects of habitat and surge. J Exp Mar Biol Ecol 105:1–20
Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term regional declines in Caribbean corals. Science 301:958–960
Hatcher BG (1982) The interaction between grazing organisms and the epilithic algal community of a coral reef: a quantitative assessment. Proc 4th Int Coral Reef Symp 2:515–524
Hay ME (1984) Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65:446–454
Hay ME (1985) Spatial patterns of herbivore impact and their importance in maintaining algal species richness. Proc 5th Int Coral Reef Symp 4:29–34
Hay ME (1991) Fish–seaweed interactions on coral reefs: effects of herbivorous fishes and adaptations of their prey. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 96–119
Hixon MA, Brostroff WN (1996) Succession and herbivory: effects of differential fish grazing on Hawaiian coral-reef algae. Ecol Monogr 66:67–90
Horn MH, Gibson RN (1990) Effects of temperature on the food processing of three species of seaweed-eating fishes from European coastal waters. J Fish Biol 37:237–247
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP, Reed DC, Boyle MJ (1987) Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J Exp Mar Biol Ecol 113:39–59
Hughes TP, Szmant AM, Steneck R, Carpenter R, Miller S (1999) Algal blooms on coral reefs: what are the causes? Limnol Oceanogr 44:1583–1586
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jaap WC (1984) The ecology of the South Florida coral reefs: a community profile. US Fish Wildl Serv, Matiere, LA FWS/OBS-82-08
Jaap WC, Halas JC, Muller RG (1988) Community dynamics of stony corals (Milleporina and Scleractinia) at Key Largo National Marine Sanctuary, Florida, during 1981–1986. Proc 6th Int Coral Reef Symp 2:237–243
Jackson JBC (1997) Reefs since Columbus. Coral Reefs 16:S23–S322
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Klumpp DW, McKinnon AD (1989) Temporal and spatial patterns in primary production of a coral-reef epilithic algal community. J Exp Mar Biol Ecol 131:1–22
Klumpp DW, Polunin NVC (1990) Algal production, grazers and habitat partitioning on a coral reef: positive correlation between grazing rate and food availability. In: Proceedings of the 24th European marine biology symposium, pp 372–388
Kramer PA (2003) Synthesis of coral reef health indicators for the western Atlantic: results of the AGRRA Program (1997–2000). In: Lang JC (ed) Status of coral reefs in the western Atlantic: results of initial surveys, Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program. Atoll Research Bulletin, pp 1–57
Lapointe BE (1997) Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol Oceanogr 42:1119–1131
Lessios H (1988) Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annu Rev Ecol Syst 19:371–393
Lessios HA (2005) Diadema antillarum populations in Panama twenty years following mass mortality. Coral Reefs 24:125–127
Lessios HA, Robertson DR, Cubit JD (1984) Spread of Diadema mass mortality through the Caribbean. Science 226:335–338
Levitan DR (1988) Algal-urchin biomass responses following the mass mortality of Diadema antillarum Philippi at Saint John, US Virgin Islands. J Exp Mar Biol Ecol 119:167–178
Lewis SM (1986) The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr 56:183–200
Lewis SM, Wainwright PC (1985) Herbivore abundance and grazing intensity on a Caribbean coral reef. J Exp Mar Biol Ecol 87:215–228
Liddell WD, Ohlhorst SL (1986) Changes in benthic community composition following the mass mortality of Diadema at Jamaica. J Exp Mar Biol Ecol 95:271–278
Matthews TR, Donahue S (1997) By-catch abundance, mortality and escape rates in wire and wooden spiny lobster traps. In: Proceedings of the 49th Gulf Caribbean Fish Institute, pp 280–298
McClanahan TR, Hendrick V, Rodrigues MJ, Polunin NVC (1999) Varying responses of herbivorous and invertebrate-feeding fishes to macroalgal reduction on a coral reef. Coral Reefs 18:195–203
McCook LJ (1999) Macroalgae, nutrients, and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18:357–367
Miller MW, Hay ME, Miller SL, Malone D, Sotka EE, Szmant AM (1999) Effects of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnol Oceanogr 44:1847–1861
Morrison D (1988) Comparing fish and urchin grazing in shallow and deeper coral reef algal communities. Ecology 69:1367–1382
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Box S, Stoffle RW, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101
Paddack MJ (2005) Herbivorous coral reef fishes in a changing ecosystem. Ph.D. thesis, University of Miami, p 151
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Pandolfi JM, Jackson JBC, Baron N, Bradbury RH, Guzman HM, Hughes TP, Kappel CV, Micheli F, Ogden JC, Possingham HP, Sala E (2005) Are U.S. coral reefs on the slippery slope to slime? Science 37:1725–1726
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres FJ (1998) Fishing down marine food webs. Science 279:860–863
Porter JW, Meier OW (1992) Quantification of loss and change in Floridian reef coral populations. Am Zool 32:625–640
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanogr 5:665–847
Robertson DR (1991) Increases in surgeonfish populations after mass mortality of the sea urchin Diadema antillarum in Panama indicate food limitation. Mar Biol 111:437–444
Russ GR, St John J (1988) Diets, growth rates, and secondary production of herbivorous coral reef fishes. Proc 6th Int Coral Reef Symp 2:37–43
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67
Sammarco PW (1986) Comparison between Atlantic and Pacific tropical marine ecosystems: community structure, ecological processes, and productivity. UNESCO/COMAR workshop 46:127–165
Seymour RJ, Tegner MJ, Dayton PK, Parnell PE (1989) Storm wave induced mortality of giant kelp, Macrocystis pyrifera, in southern California. Estuar Coast Shelf S 28:277–292
Shinn EA (1988) The geology of the Florida Keys. Oceanus 31:46–53
Smith SV, Kimmerer WJ, Laws EA, Brock RE, Walsh TW (1981) Kaneohe Bay sewage diversion experiment: perspectives on ecosystem responses to nutritional perturbation. Pac Sci 35:279–385
Steneck RS (1983) Quantifying herbivory on coral reefs: just scratching the surface and still biting off more than we can chew. Symp Ser Undersea Res 1:103–111
Steneck RS (1988) Herbivory on coral reefs: a synthesis. In: Proceedings of the 6th international coral reef symposium, vol 1, pp 37–49
Steneck RS, Dethier MN (1994) A functional group approach to the structure of algal-dominated communities. Oikos 69:476–498
Sutherland DL, Harper DE (1983) The wire fish-trap fishery of Dade and Broward counties, Florida December 1979–September 1980. Marine Research Laboratory, FL, USA
Szmant AM, Forrester A (1996) Water column and sediment nitrogen and phosphorus distribution patterns in the Florida Keys, USA. Coral Reefs 15:21–41
van Rooij JM, Videler J, Bruggemann JH (1998) High biomass and production but low energy transfer efficiency of Caribbean parrotfish: implications for trophic models of coral reefs. J Fish Biol 53:154–1778
Wanders JBW (1976) The role of benthic algae in the shallow reef of Curacao (Netherlands Antilles). I: Primary productivity in the coral reef. Aquat Bot 2:235–270
Williams ID, Polunin NVC (2001) Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19:358–366
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol-Prog Ser 222:187–196
Acknowledgments
Funding was provided by the National Center for Caribbean Coral Reef Research (NCORE) through EPA grant #R828020. R.S. Steneck, M.M. Miller, and J.M. McManus provided valuable comments on the project and paper. We are extremely grateful to many field assistants: K. Grorud-Colvert, A.L. Chapin, M.C. Sullivan, C. Paris, K. Huebert, C. Boynton, T. Rankin, L. Buhrmaster, A. Miyake, C. Dickman, W. Cooper, J. Kool, D. Richardson, J. Llopiz, M. Brandt, A. Yniquez, K. Denit, D. Pinkard, C. Guigand, S. Cappell, J. Fortuna, T. Smith, J. Yoshina, N. Sbeih. The comments of three anonymous reviewers greatly improved this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor M.I. McCormick
Rights and permissions
About this article
Cite this article
Paddack, M.J., Cowen, R.K. & Sponaugle, S. Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 25, 461–472 (2006). https://doi.org/10.1007/s00338-006-0112-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-006-0112-y