Abstract
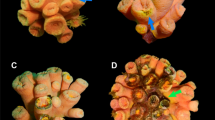
Unusual disease lesions were observed in Montipora corals on the fringing reef of Magnetic Island (Great Barrier Reef, Australia) following a period of high water temperature in early January 2002. Tissue death in Montipora spp. appeared as a black layer that spread rapidly across the colony surface, though this appeared as the final phase of disease progression (with three previous disease phases now identified, S. Anthony, unpublished). Culture and molecular-based microbial analysis of this layer did not identify a likely microbial pathogen. Despite this, DNA sequencing of microbial 16S rDNA indicated a shift in the bacterial population associated with affected coral tissue. A clone library of the healthy coral sample predominantly contained sequences within the γ-Proteobacteria. A disease coral sample representing the margin of the black lesion and healthy coral tissue was dominated by sequences, which demonstrated low sequence identity to a range of α-Proteobacteria, γ-Proteobacteria and cyanobacteria. The microbes identified in the diseased Montipora spp. samples are likely to be opportunistic rather than the causative agent of the observed lesion. Studies are in progress to further characterise the ecology of this disease and describe the potential microbial pathogen(s).



Similar content being viewed by others
References
Altschul SF, Madden TL, Schaeffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Asahida T, Kobayashi T, Saitoh K, Nakayama I (1996) Tissue preservation and total DNA extraction from fish stored at ambient temperature using buffers containing high concentrations of urea. Fish Sci 62:727–730
Banin E, Israely T, Kushmaro A, Loya Y, Orr E, Rosenberg E (2000) Penetration of the coral-bleaching bacterium Vibrio shiloi into Oculina patagonica. Appl Environ Microbiol 66:3031–3036
Ben-Haim Y, Rosenberg E (2002) A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar Biol 141:47–55
Ben-Haim Y, Banin E, Kushmaro A, Loya Y, Rosenberg E (1999) Inhibition of photosynthesis and bleaching of zooxanthellae by the coral pathogen Vibrio shiloi. Environ Microb 1:223–229
Berkelmans R, De’ath G, Kininmonth S, Skirving WJA (2004) Comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: Spatial correlation, patterns and predictions. Coral Reefs 23:74–83
Bolinches J, Romalde JL, Toranzo AE (1988) Evaluation of selective media for isolation and enumeration of vibrios from estuarine waters. J Microbiol Methods 8:151–160
Bryant D, Burke L, McManus J, Spalding M (1998) Reefs at Risk: a map-based indicator of threats to the world’s coral reefs. World Resources Institute, p 56
Chao A (1984) Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Colwell RK (1997) EstimateS: statistical estimate of species richness and shared species from samples. Version 5. http://viceroy.eeb.uconn.edu/estimates
Cooney RP, Pantos O, Le Tissier MDA, Barer MR, O’Donnell AG, Bythell JC (2002) Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environ Microbiol 4:401–413
Denner EB, Smith GW, Busse HJ, Schumann P, Narzt T, Polson SW, Lubitz W, Richardson LL (2003) Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int J Syst EvolMicrobiol 53:1115–1122
Fisher RA, Corber AS, Williams CB (1943) The relation between the number of species and the number of individuals in a random sample of an animal population. J Anim Ecol 12:42–58
Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW (2002) Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl Environ Microbiol 68:2214–2228
Giovannoni S, Rappé M (2000) Evolution, diversity and molecular ecology of marine prokaryotes. In: Kirchman D (ed) Microbial ecology of the oceans. Wiley-Liss Inc., New York, pp 47–84
Gladfelter E (1982) White-band disease in Acropora palmata: implication for the structure and growth of shallow reefs. Bull Mar Sci 32:639–643
Good IJ (1953) The population frequencies of species and the estimation to the population parameters. Biometrika 40:237–264
Harvell CD, Kim K, Burkholder J, Colwell R, Epstein P, Grimes D, Hofmann E, Lipp E, Osterhaus A, Overstreet R, Porter J, Smith G, Vasta G (1999) Emerging marine diseases—climate links and anthropogenic factors. Science 285:1505–1510
Heck KL Jr, Van Belle G, Simberloff D (1975) Explicit calculation of the rarefaction diversity measurements and the determination of sufficient sample size. Ecology 56:1459–1461
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshwater Res 50:839–866
Holland SH (1998) A RarefactWin program, version 1.2. University of Georgia, Athens. http://www.uga.edu/strata/Software.html
Hugenholtz P, Goebel BM, Pace NR (1998) Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180:4765–4774
Hurlbert SH (1971) The non-concept of species diversity: a critique and alternative parameters. Ecology 52:577–586
Jones RJ, Bowyer J, Hoegh-Guldberg O, Blackall LL (2004) Dynamics of a temperature-related coral disease outbreak. Mar Ecol Progr Ser (in press)
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 177–203
Ludwig W, Strunk O, Klugbauer S, Klugbauer N, Weizenegger M, Neumaier J (1998) Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554–568
Magurran AE (1998) Ecological diversity and its measurement. Princeton University Press, Princeton
Maidak BL, Olsen GJ, Larsen N, Overbeek R, McCaughey MJ, Woese CR (1996) The Ribosomal Database Project (RDP). Nucleic Acids Res 24:82–85
Moyer C, Dobbs F, Karl D (1994) Estimation of diversity and community structure through restriction fragment length polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol 60:871–879
Pantos O, Cooney RP, Le Tissier MDA, Barer MR, O’Donnell AG, Bythell JC (2003) The bacterial ecology of a plague-like disease affecting the Caribbean coral Montastrea annularis. Environ Microbiol 5:370–382
Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavvy DL, Smith GW (2002) The aetiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. PNAS 99:8725–8730
Porter J, Dunstan P, Jaap W, Patterson K, Kosmynin V, Meier O, Patterson M, Parsons M (2001) Patterns of spread of coral disease in the Florida Keys. Hydrobiologia 460:1397–1407
Richardson LL (1996) Horizontal and vertical migration patterns of Phormidium corallyticum and Beggiatoa spp associated with black band disease of corals. Microbial Ecol 32:323–335
Richardson LL (1998) Coral diseases: what is really known? Trends Ecol Conservation 13:438–443
Richardson LL, Kuta KG, Schnell S, Carlton RG (1997) Ecology of the black band disease microbial consortium, In: Proceedings of the 8th International Coral Reef Symposium, vol 1. Balboa, Panama, pp 597–600
Richardson LL, Goldberg WM, Kuta KG, Aronson RB, Smith GW, Ritchie KB (1998) Florida’s mystery coral-killer identified. Nature 392:557–558
Ritchie KB, Smith GW (1995) Preferential carbon utilization by surface bacterial communities from water mass, normal, and white-band diseased Acropora cervicornis. Mol Mar Biol Biotechnol 4:345–354
Ritchie KB, Smith GW (1997) Physiological comparison of bacterial communities from various species of scleractinian corals. In: Proceedings of the 8th international coral reef symposium vol 1. Balboa, Panama 1:521–526
Rohwer F, Breitbart M, Jara J, Azam F, Knowlton N (2001) Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs 20:85–91
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10
Rohwer F, Kelley S (2004) Culture-independent analyses of coral-associated microbes. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer-Verlag, New York, pp 265–277
Rosenberg E, Ben-Haim Y (2002) Microbial diseases of corals and global warming. Environ Microbiol 4(6):318–326
Rützler K, Santavy DL (1983) The black band disease of Atlantic reef corals. I. Description of the cyanophyte pathogen. Mar Ecol 4:301–319
Santavy DL (1995) The diversity of microorganisms associated with marine invertebrates and their roles in the maintenance of ecosystems. In: Allsopp D, Colwell RR, Hawksworth D L (eds) Microbial diversity and ecosystem function. CAB International, Wallingford, pp 211–229
Saunders SE, Burke JF (1990) Rapid isolation of miniprep DNA for double stranded sequencing. Nucleic Acids Res 18:4948
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Shashar N, Cohen Y, Loya Y, Sar N (1994) Nitrogen fixation (acetylene reduction) in stony corals: evidence for coral bacterial interactions. Mar Ecol Prog Ser 111:259–264
Simberloff D (1978) Use of rarefaction and related methods in ecology. In: Dickson KL, Cairns JJ, Livingston RJ (eds) Biological data in water pollution assessment: quantitative and statistical analyses. American Society for Testing and Materials, Philadelphia, pp 150–165
Simpson EH (1949) Measurement of diversity. Nature 163:668
Strunk O, Gross O, Reichel B, May M, Herman S, Stuckman N, Nonhoff B, Lenke M, Ginhart A, Vilbig A, Ludwig T, Bode A, Schleifer K-H, Ludwig W (1998) ARB: a software environment for sequence data. Department of Microbiology Technische Inversitat Munchen, Munich,
Ward DM (1998) A natural species concept for prokaryotes. Curr Opin Microbiol 1:271–277
Webster NS, Hill RT (2001) The culturable microbial community of the Great Barrier Reef sponge Rhopaloeides odorabile is dominated by an α-Proteobacterium. Mar Biol 138:843–851
Webster NS, Negri AP, Webb RI, Hill RT (2002) A spongin-boring α-Proteobacterium is the etiological agent of disease in the Great Barrier Reef sponge Rhopaloeides odorabile. Mar Ecol Prog Ser 232:305–309
Williams WM, Viner AB, Broughton WJ (1987) Nitrogen fixation (acetylene reduction) associated with living coral Acropora variablis . Mar Biol 94:531–535
Willis BL, Page CA, Dinsdale EA (2004) Coral Diseases on the Great Barrier Reef. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer-Verlag, New York, pp 69–104
Acknowledgements
I would like to thank Carolyn Smith, Mary Wakeford, and Ray Berkelmans for field work and helpful discussions. Also, the author is indebted to Nicole Webster and Tim Simmonds for comments and help in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biological Editor H.R. Lasker
Rights and permissions
About this article
Cite this article
Bourne, D.G. Microbiological assessment of a disease outbreak on corals from Magnetic Island (Great Barrier Reef, Australia). Coral Reefs 24, 304–312 (2005). https://doi.org/10.1007/s00338-005-0479-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-005-0479-1