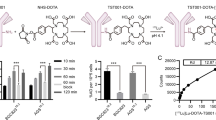
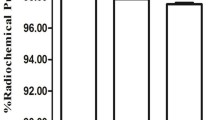
Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is a highly malignant solid tumor that lacks early diagnostic methods. Recently, targeted immunotherapy and radiotherapy have been integrated with radionuclide-antibody conjugate drugs, which can be used for targeted diagnosis and dynamic imaging of tumors. CEACAM6 is overexpressed in pancreatic tumors and is a potential theranostic target for PDAC. We aimed to develop a novel targeted carrier for theranostics of PDAC and other solid tumors.
Methods
Based on camelid heavy-chain-only antibodies, we developed a CEACAM6-targeting recombinant antibody NY004, and evaluated it as a novel antibody-carrier for imaging and therapy of cancer in tumor models. We labeled NY004 with theranostic nuclides and applied this self-developed antibody platform in diagnostic imaging and antitumor assessment in PDAC models.
Results
Through microPET, IHC, and biodistribution assays, targeting and biodistribution of [89Zr]-NY004 in solid tumors including PDAC was examined, and the investigated tumors were all CEACAM6-positive malignancies. We found that NY004 was suitable for use as a drug carrier for radioimmunotheranostics. Our study showed that NY004 was characterized by high targeted uptake and a long retention time in PANC-1 tumors (up to 6 days post-injection), with good specificity and high imaging efficiency. Therapeutic evaluation of the radionuclide-labeled antibody drug [177Lu]-NY004 in PDAC tumor-bearing model revealed that NY004 had high and prolonged uptake in tumors, relatively low non-target organ uptake, and good anti-tumor efficacy.
Conclusion
As a drug platform for radiotheranostics, CEACAM6-specific antibody NY004 met the requirements of easy-labeling, targeting specificity, and effective persistence in pancreatic adenocarcinoma tissues.
Key Points
• [89Zr]-NY004 has good specificity and high imaging efficiency, and is characterized by high tumor-targeting uptake and a long tumor retention time as a PET molecular imaging tracer.
• Therapeutic radionuclide-conjugated antibody drug [177Lu]-NY004 has high uptake and prolonged uptake duration in tumors, low non-target organ uptake, and significant tumor-inhibiting efficacy in PDAC model.
• The self-developed antibody structure NY004 is a promising drug platform for radioimmunotheranostics of CEACAM6-positive tumors including pancreatic ductal adenocarcinoma.






Similar content being viewed by others
Abbreviations
- ADC:
-
Antibody-drug conjugate
- CEA:
-
Carcinoembryonic antigen
- CEACAM6:
-
Carcinoembryonic antigen–related cell adhesion molecule-6
- ECM:
-
Extracellular matrix
- Fc:
-
Crystallizable fragment
- IHC:
-
Immuno-histochemical
- PDAC:
-
Pancreatic ductal adenocarcinoma
- RAC:
-
Radionuclide-antibody conjugate
- RIT:
-
Radioimmuno-therapy
References
Uddin MH, Al-Hallak MN, Philip PA et al (2021) Exosomal microRNA in pancreatic cancer diagnosis, prognosis, and treatment: from bench to bedside. Cancers (Basel). 13(11):2777. https://doi.org/10.3390/cancers13112777
Yoon JH, Jung YJ, Moon SH (2021) Immunotherapy for pancreatic cancer. World J Clin Cases 9(13):2969–2982. https://doi.org/10.12998/wjcc.v9.i13.2969
Williamson T, de Abreu MC, Trembath DG et al (2021) Mebendazole disrupts stromal desmoplasia and tumorigenesis in two models of pancreatic cancer. Oncotarget 12(14):1326-1338. https://doi.org/10.18632/oncotarget.28014
Latenstein AEJ, van der Geest LGM, Bonsing BA et al (2020) Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer 125:83-93. https://doi.org/10.1016/j.ejca.2019.11.002
Gupta N, Yelamanchi R (2021) Pancreatic adenocarcinoma: a review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J Gastroenterol 27(23):3158–3181. https://doi.org/10.3748/wjg.v27.i23.3158
Zeeshan MS, Ramzan Z (2021) Current controversies and advances in the management of pancreatic adenocarcinoma. World J Gastrointest Oncol 13(6):472–494. https://doi.org/10.4251/wjgo.v13.i6.472
Herrmann K, Schwaiger M, Lewis JS et al (2020) Radiotheranostics: a roadmap for future development. Lancet Oncol 21(3):e146–e156. https://doi.org/10.1016/S1470-2045(19)30821-6
Hafeez U, Parakh S, Gan HK, Scott AM (2020) Antibody-drug conjugates for cancer therapy. Molecules 25(20):4764. https://doi.org/10.3390/molecules25204764
Joubert N, Beck A, Dumontet C, Denevault-Sabourin C (2020) Antibody-drug conjugates: the last decade. Pharmaceuticals (Basel) 13(9):245. https://doi.org/10.3390/ph13090245
Drago JZ, Modi S, Chandarlapaty S (2021) Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol 18(6):327–344. https://doi.org/10.1038/s41571-021-00470-8
Xiang W, Lv Q, Shi H, Xie B, Gao L (2020) Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta 214:120716. https://doi.org/10.1016/j.talanta.2020.120716
Van Manen L, Groen JV, Putter H et al (2020) Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers 25(2):186–193. https://doi.org/10.1080/1354750X.2020.1725786
Hayakawa H, Fukasawa M, Sato T et al (2019) Carcinoembryonic antigen level in the pancreatic juice is effective in malignancy diagnosis and prediction of future malignant transformation of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol 54(11):1029–1037. https://doi.org/10.1007/s00535-019-01592-8
Cameron S, de Long LM, Hazar-Rethinam M et al (2012) Focal overexpression of CEACAM6 contributes to enhanced tumourigenesis in head and neck cancer via suppression of apoptosis. Mol Cancer 11:74. https://doi.org/10.1186/1476-4598-11-74.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P (2012) Anoikis: an emerging hallmark in health and diseases. J Pathol 226(2):380–393. https://doi.org/10.1002/path.3000
Holmer R, Wätzig GH, Tiwari S, Rose-John S, Kalthoff H (2015) Interleukin-6 trans-signaling increases the expression of carcinoembryonic antigen-related cell adhesion molecules 5 and 6 in colorectal cancer cells. BMC Cancer 15:975. https://doi.org/10.1186/s12885-015-1950-1
Chen J, Li Q, An Y et al (2013) CEACAM6 induces epithelial-mesenchymal transition and mediates invasion and metastasis in pancreatic cancer. Int J Oncol 43(3):877–885. https://doi.org/10.3892/ijo.2013.2015
Cheng TM, Murad YM, Chang CC et al (2014) Single domain antibody against carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) inhibits proliferation, migration, invasion and angiogenesis of pancreatic cancer cells. Eur J Cancer 50(4):713–721. https://doi.org/10.1016/j.ejca.2012.07.019
Pandey R, Zhou M, Islam S et al (2019) Carcinoembryonic antigen cell adhesion molecule 6 (CEACAM6) in pancreatic ductal adenocarcinoma (PDA): an integrative analysis of a novel therapeutic target. Sci Rep 9(1):18347. https://doi.org/10.1038/s41598-019-54545-9
Lindner T, Loktev A, Altmann A et al (2018) Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med 59(9):1415–1422. https://doi.org/10.2967/jnumed.118.210443
Watabe T, Liu Y, Kaneda-Nakashima K et al (2020) Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J Nucl Med 61(4):563–569. https://doi.org/10.2967/jnumed.119.233122
Cheng JC, Klausen C, Leung PC (2013) Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett 329(2):197–206. https://doi.org/10.1016/j.canlet.2012.10.029
McDonald PC, Chafe SC, Brown WS et al (2019) Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology 157(3):823–837. https://doi.org/10.1053/j.gastro.2019.05.004
Miar A, Arnaiz E, Bridges E et al (2020) Hypoxia induces transcriptional and translational downregulation of the type I IFN pathway in multiple cancer cell types. Cancer Res 80(23):5245–5256. https://doi.org/10.1158/0008-5472.CAN-19-2306
Ding XC, Wang LL, Zhang XD et al (2021) The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J Hematol Oncol 14(1): 92. https://doi.org/10.1186/s13045-021-01102-5
Amrutkar M, Aasrum M, Verbeke CS, Gladhaug IP (2019) Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer 19(1):596. https://doi.org/10.1186/s12885-019-5803-1
Kim SK, Jang SD, Kim H, Chung S, Park JK, Kuh HJ (2020) Phenotypic heterogeneity and plasticity of cancer cell migration in a pancreatic tumor three-dimensional culture model. Cancers (Basel) 12(5):1305. https://doi.org/10.3390/cancers12051305
Gunderson AJ, Yamazaki T, McCarty K et al (2019) Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS One 14(2):e0211117. https://doi.org/10.1371/journal.pone.0211117
Giesel FL, Kratochwil C, Lindner T et al (2019) 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 60(3):386–392. https://doi.org/10.2967/jnumed.118.215913
Meyer C, Dahlbom M, Lindner T et al (2020) Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med 61(8):1171–1177. https://doi.org/10.2967/jnumed.119.236786
Tijink BM, Perk LR, Budde M et al (2009) (124)I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of (131)I-L19-SIP radioimmunotherapy. Eur J Nucl Med Mol Imaging 36(8):1235–1244. https://doi.org/10.1007/s00259-009-1096-y
Jailkhani N, Ingram JR, Rashidian M et al (2019) Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc Natl Acad Sci U S A 116(28):14181–14190. https://doi.org/10.1073/pnas.1817442116
Vivier D, Sharma SK, Adumeau P, Rodriguez C, Fung K, Zeglis BM (2019) The impact of FcγRI binding on immuno-PET. J Nucl Med 60(8):1174–1182. https://doi.org/10.2967/jnumed.118.223636
Vivier D, Fung K, Rodriguez C et al (2020) The influence of glycans-specific bioconjugation on the FcγRI binding and in vivo performance of 89Zr-DFO-pertuzumab. Theranostics 10(4):1746–1757. https://doi.org/10.7150/thno.39089
Erba PA, Sollini M, Orciuolo E et al (2012) Radioimmunotherapy with radretumab in patients with relapsed hematologic malignancies. J Nucl Med 53(6):922–927. https://doi.org/10.2967/jnumed.111.101006
Waseem N, Aparici CM, Kunz PL (2019) Evaluating the role of theranostics in grade 3 neuroendocrine neoplasms. J Nucl Med 60(7):882–891. https://doi.org/10.2967/jnumed.118.217851
Yadav MP, Ballal S, Bal C et al (2020) Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med 45(1):19–31. https://doi.org/10.1097/RLU.0000000000002833
Tang C, Welsh JW, de Groot P et al (2017) Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 23(6):1388-1396. https://doi.org/10.1158/1078-0432.CCR-16-1432
Ozpiskin OM, Zhang L, Li JJ (2019) Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 9(5):1215–1231. https://doi.org/10.7150/thno.32648
Chen H, Zhao L, Fu K et al (2019) Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 9(25):7948–7960. https://doi.org/10.7150/thno.39203
Doctor A, Seifert V, Ullrich M, Hauser S, Pietzsch J (2020) Three-dimensional cell culture systems in radiopharmaceutical cancer research. Cancers (Basel) 12(10):2765. https://doi.org/10.3390/cancers12102765
Zang M, Zhang Y, Zhang B et al (2015) CEACAM6 promotes tumor angiogenesis and vasculogenic mimicry in gastric cancer via FAK signaling. Biochim Biophys Acta 1852(5):1020–1028. https://doi.org/10.1016/j.bbadis.2015.02.005
Zhu R, Ge J, Ma J, Zheng J (2019) Carcinoembryonic antigen related cell adhesion molecule 6 promotes the proliferation and migration of renal cancer cells through the ERK/AKT signaling pathway. Transl Androl Urol 8(5):457–466. https://doi.org/10.21037/tau.2019.09.02
Shan L 124I-Labeled anti-prostate stem cell antigen affinity-matured A11 minibody. 2010 Jun 23 [updated 2010 Jul 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013
Muyldermans S (2013) Nanobodies: natural single-domain antibodies. Annu Rev Biochem 82:775–797. https://doi.org/10.1146/annurev-biochem-063011-092449
Ingram JR, Schmidt FI, Ploegh HL (2018) Exploiting nanobodies’ singular traits. Annu Rev Immunol 36:695–715. https://doi.org/10.1146/annurev-immunol-042617-053327
Acknowledgements
We gratefully acknowledge Chi Lai Ho for reviewing the manuscript for critical comments.
Funding
This study has received funding from the Original Research Personalized Support Project, Fudan University (No. IDF151039/020), National Natural Science Foundation of China (No. 81701732, 82071962), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), and ZJLab, Shanghai Municipal Key Clinical Specialty (shslczdzk03402) and Clinical Research Plan of SHDC (No. SHDC2020CR2056B).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yihui Guan.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Yanyan Kong kindly provided statistical advice for this manuscript. One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Approval from the institutional animal care committee was obtained.
Ethical approval
Institutional review board approval was obtained. All animal studies were performed in accordance with the guidelines of the Animal Welfare Office of Fudan University. The Institutional Animal Care and Use Committee of Fudan University reviewed and approved the experimental procedures. The animals were maintained under anesthesia during the model development, injection, accumulation, and scanning periods to avoid suffering at each stage of the experiment.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
• experimental study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, Y., Xie, F., Zhang, Z. et al. Evaluation of novel anti-CEACAM6 antibody-based conjugates for radioimmunotheranostics of pancreatic ductal adenocarcinoma. Eur Radiol 33, 7077–7088 (2023). https://doi.org/10.1007/s00330-023-09679-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09679-w