Abstract
Objectives
High-resolution post-contrast T1-weighted imaging is a workhorse sequence in the evaluation of neurological disorders. The T1-MPRAGE sequence has been widely adopted for the visualization of enhancing pathology in the brain. However, this three-dimensional (3D) acquisition is lengthy and prone to motion artifact, which often compromises diagnostic quality. The goal of this study was to compare a highly accelerated wave-controlled aliasing in parallel imaging (CAIPI) post-contrast 3D T1-MPRAGE sequence (Wave-T1-MPRAGE) with the standard 3D T1-MPRAGE sequence for visualizing enhancing lesions in brain imaging at 3 T.
Methods
This study included 80 patients undergoing contrast-enhanced brain MRI. The participants were scanned with a standard post-contrast T1-MPRAGE sequence (acceleration factor [R] = 2 using GRAPPA parallel imaging technique, acquisition time [TA] = 5 min 18 s) and a prototype post-contrast Wave-T1-MPRAGE sequence (R = 4, TA = 2 min 32 s). Two neuroradiologists performed a head-to-head evaluation of both sequences and rated the visualization of enhancement, sharpness, noise, motion artifacts, and overall diagnostic quality. A 15% noninferiority margin was used to test whether post-contrast Wave-T1-MPRAGE was noninferior to standard T1-MPRAGE. Inter-rater and intra-rater agreement were calculated. Quantitative assessment of CNR/SNR was performed.
Results
Wave-T1-MPRAGE was noninferior to standard T1-MPRAGE for delineating enhancing lesions with unanimous agreement in all cases between raters. Wave-T1-MPRAGE was noninferior in the perception of noise (p < 0.001), motion artifact (p < 0.001), and overall diagnostic quality (p < 0.001).
Conclusion
High-accelerated post-contrast Wave-T1-MPRAGE enabled a two-fold reduction in acquisition time compared to the standard sequence with comparable performance for visualization of enhancing pathology and equivalent perception of noise, motion artifacts and overall diagnostic quality without loss of clinically important information.
Key Points
• Post-contrast wave-controlled aliasing in parallel imaging (CAIPI) T1-MPRAGE accelerated the acquisition of three-dimensional (3D) high-resolution post-contrast images by more than two-fold.
• Post-contrast Wave-T1-MPRAGE was noninferior to standard T1-MPRAGE with unanimous agreement between reviewers (100% in 80 cases) for the visualization of intracranial enhancing lesions.
• Wave-T1-MPRAGE was equivalent to the standard sequence in the perception of noise in 94% (75 of 80) of cases and was preferred in 16% (13 of 80) of cases for decreased motion artifact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contrast enhanced T1-weighted imaging of the brain is essential for comprehensive evaluation of a wide range of inflammatory, neoplastic, and neurovascular diseases of the central nervous system. Although several MRI techniques can detect disruption of the blood-brain barrier after contrast injection, the magnetization-prepared rapid gradient echo (MPRAGE) sequence has been used in clinical neuroimaging for decades [1]. Despite reports that post-contrast three-dimensional (3D) T1-weighted spin echo sequences such as SPACE offer better conspicuity of enhancing lesions [2] and leptomeningeal abnormalities [3], the incomplete suppression of slow-flowing blood may confound the detection of intracranial enhancing lesions, including the misdiagnosis of intraparenchymal veins as multiple sclerosis lesions [4]. As such, T1-MPRAGE remains the most commonly used 3D T1-weighted post-contrast sequence used in clinical neuroimaging, as evidenced by its inclusion in several major consensus recommendations for imaging of brain tumors and metastases [5, 6], multiple sclerosis [7], and epilepsy [8]. Due to its high isotropic resolution, 3D T1-MPRAGE provides exquisite 3D visualization of enhancing pathology for guiding biopsy, surgical resection, and treatment planning, as well as precise delivery of stereotactic radiation. However, the long scan time (~5–6 min) and motion sensitivity of the 3D encoding often degrades the image quality, thereby compromising the visualization of small enhancing lesions and decreasing the overall diagnostic yield of this sequence. Therefore, new approaches to accelerating the T1-MPRAGE sequence for evaluation of intracranial enhancing lesions would be highly beneficial and see significant impact across a wide range of protocols in neuroradiology.
The wave-controlled aliasing in parallel imaging (Wave-CAIPI) acquisition and reconstruction technique is a fast imaging approach that provides up to an order of magnitude of acceleration compared to standard parallel imaging [9]. Wave-CAIPI efficiently encodes 3D k-space by synergistically combining CAIPI shifts along ky/kz with a corkscrew trajectory along the readout (kx), resulting in voxel aliasing along all three spatial dimensions. Wave-CAIPI takes full advantage of the 3D coil sensitivity information when using high-channel count array coils to provide high acceleration factors with negligible noise amplification across a variety of contrasts. Prior studies have demonstrated the benefits of Wave-CAIPI encoding in decreasing scan time and motion artifact while preserving diagnostic quality in specific clinical scenarios, including susceptibility-weighted imaging for the detection of hemorrhage [10], noncontrast T1-weighted MPRAGE for the assessment of cortical volumes [11], fluid attenuated inversion recovery imaging for detection of white matter lesions [12], and post-contrast T1-weighted SPACE for detection of enhancing pathology [13, 14]. Incorporating Wave-CAIPI acceleration into post-contrast 3D T1-MPRAGE imaging would increase the accessibility and yield of high-resolution post-contrast imaging, particularly in motion-prone patients, and merits systematic validation in a realistic clinical environment.
The goal of this study was to compare a highly accelerated Wave-CAIPI post-contrast 3D T1-MPRAGE sequence (Wave-T1-MPRAGE) with the standard high-resolution 3D T1-MPRAGE sequence for routine clinical brain imaging with contrast at 3 T. We hypothesized that Wave-T1-MPRAGE would be noninferior to the standard sequence accelerated using GRAPPA (generalized autocalibrating partially parallel acquisition) parallel imaging in visualizing enhancing lesions and would provide equivalent diagnostic quality while reducing the acquisition time by more than half.
Materials and methods
Study participants
This single-center retrospective study was approved by the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Given that the additional scan time from the Wave-T1-MPRAGE sequence was less than 3 min per scan, the institutional review board waived the need for signed informed consent. Instead, an information sheet presenting a detailed description of the research study was provided to the study participants, who could decline participation in the study prior to initiating their scan.
Between February and May of 2020, a total of 96 consecutive patients undergoing contrast-enhanced brain MRI at the Massachusetts General Hospital were scanned as part of the study. Inclusion criteria were referral for contrast-enhanced brain MRI as an inpatient or outpatient and assent to participation in the study. Patients could not receive a contrast-enhanced brain MRI if they had compromised renal function (i.e., eGFR < 30 mL/min/1.73 m2) resulting in inability to receive contrast, claustrophobia, or other contraindications to MRI.
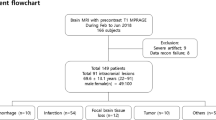
Datasets were then reviewed for inclusion in the retrospective analysis of image quality. Datasets were excluded from our retrospective analysis if one of the sequences for evaluation (Standard vs Wave-T1-MPRAGE) was missing, if contrast extravasation occurred during the examination, or if the sequence parameter were not well matched. A flowchart of the screening process for inclusion in the comparative evaluation study is provided in Fig. 1.
MRI protocol
Whole-brain MRI scans were performed on a 3-T MRI system (MAGNETOM Prisma, Siemens Healthineers) using a commercially available 20- or 32-channel receiver coil array, which was chosen for best patient fit by the scanning MR technologist. In addition to the conventional structural sequences for each clinical indication (T1-weighted, T2-weighted, FLAIR, diffusion-weighted, and susceptibility-weighted images), the imaging protocol of all MRI studies included a standard post-contrast T1-MPRAGE sequence (acceleration factor [R] = 2 using GRAPPA parallel imaging, acquisition time [TA] = 5 min 18 s, 0.9 mm isotropic spatial resolution) and a prototype post-contrast Wave-CAIPI T1-MPRAGE sequence (R = 4, TA = 2 min 32 s, 1.0 mm isotropic spatial resolution) with comparable effective spatial resolution. The “standard” T1 MPRAGE sequence/protocol was implemented by the clinical MRI physics team on the 3-T scanners using the default parameters suggested by the vendor (e.g., TR = 2300 ms, TI = 900 ms, shortest possible TE) and are similar to those suggested by several consensus recommendations [6]. The Wave-CAIPI T1 MPRAGE protocol was created following the “standard” MPRAGE protocol to achieve comparable contrast and spatial resolution, with matched TR, TI, TE and flip angle values. Contrast-enhanced images were obtained after intravenous administration of standard dose of 0.2 mL/kg (0.1 mmol/kg) of gadoterate meglumine (Dotarem®, Guerbet) at a flow rate of approximately 2 mL/s. To minimize potential differences related to the order of acquisition, the order of the standard and Wave-T1-MPRAGE sequences was switched halfway through the study, as done in previous studies to mitigate against bias introduced by differences in timing of imaging after contrast administration.
Post-contrast wave-CAIPI T1-MPRAGE sequence acquisition and reconstruction
The standard T1-MPRAGE sequence used in our institution’s routine clinical protocol employs the default vendor reconstruction filter that introduces a small degree of spatial smoothing. The Wave-T1-MPRAGE sequence is currently only available as a works-in-progress prototype and as such does not yet have spatial filtering incorporated as an option. To overcome the constraints imposed by the vendor on our post-processing options, we performed a systematic evaluation of image sharpness using different effective resolutions (1 mm isotropic versus 0.9 mm isotropic) for both the standard and Wave T1-MPRAGE sequences and found that 1 mm isotropic voxel size for Wave T1-MPRAGE as compared to 0.9 mm isotropic voxel size for standard T1-MPRAGE (which incorporated spatial filtering by default) provided the most comparable effective spatial resolution. Therefore, a marginally larger isotropic voxel size was used in the Wave-T1-MPRAGE compared to the standard T1-MPRAGE acquisitions (1.0 mm vs 0.9 mm). The TR, TE, and flip angle values were matched between the Wave and standard T1-MPRAGE sequences as those are the main parameters that contribute to T1-weighted contrast. These strategies ensured comparable contrast and visual spatial resolution as evaluated by the study neuroradiologists. On-line reconstruction of the Wave-CAIPI 3D T1-MPRAGE was performed using an auto-calibrated procedure in which the true gradient trajectory is estimated during the reconstruction without the need for additional calibration scans [15]. This allowed for simultaneous estimation of the parallel imaging reconstruction and the true k-space trajectory, with a reconstruction time of approximately 60 s. Detailed sequence parameters are presented in Table 1.
Image evaluation
Two neuroradiologists (C.N. and S.Y.H.; 14 and 11 years of experience, respectively) performed a randomized independent review of all contrast-enhanced images. All raters were blinded to sequence type, clinical indication and other imaging test results. The post-contrast images of the anonymized DICOM datasets were evaluated on an independent workstation. A predetermined 5-point grading scale was used to compare Wave-T1-MPRAGE with standard T1-MPRAGE in the evaluation of intracranial enhancement (Electronic Supplementary Table). The Wave-T1-MPRAGE and standard T1-MPRAGE images were evaluated in a head-to-head comparison for the detection of abnormal enhancement in the parenchyma, leptomeninges, pachymeninges (dura), and ependymal surface. The reviewers rated the images on an independent workstation and were allowed to choose the windowing levels for optimal visualization of contrast enhancement on both sequences. The window level (WL) and width (WW) settings may slightly differ for each sequence as they use fundamentally different acquisition and reconstruction techniques, thereby making it impossible to match the exact values of WW/WL. For full transparency, we have reported the window width and level settings in each panel of Figs. 2 and 3. Reviewers also evaluated the presence of artifacts related to motion, degree of background noise, and overall diagnostic quality of the image series. In addition, for a more objective assessment of the image quality, the reviewers compared the sharpness of both sequences. Measurements of signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were also performed in each image series as described below. The order of the cases and the left/right position of each sequence on the screen were randomized. All cases were rated for each feature with the 5-point grading scale, in which positive numbers favored the sequence on the right, and negative numbers favored the sequence on the left of the screen. A third neuroradiologist with over 20 years of experience (O.R.) adjudicated cases in which the reviewers differed by > 1 point in their ratings. We chose not to have the third radiologist rate all the cases in order to avoid complicating the statistical analysis, which would have been more complex with three raters.
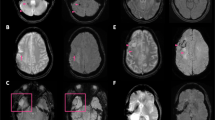
Head-to-head comparison of post-contrast Wave-CAIPI T1-MPRAGE and standard T1-MPRAGE sequences. A Axial contrast-enhanced images of a 43-year-old man with a glioblastoma in the left frontal and temporal lobes. B Sagittal contrast-enhanced images of a 71-year-old man with brain metastases from non-small cell lung cancer. Window width (WW) and window level (WL) are reported alongside each image
Head-to-head comparison of post-contrast Wave-CAIPI T1-MPRAGE and standard T1-MPRAGE sequences. A Coronal contrast-enhanced images of a 74-year-old woman presenting with a dural enhancing lesion compatible with a meningioma along the right parietal convexity. B Axial contrast-enhanced images of a 52-year-old woman with non-small cell lung cancer showing tiny enhancing metastatic lesions in the left cerebellar hemisphere (arrow). Window width (WW) and window level (WL) are reported alongside each image
Quantitative evaluation
Quantitative evaluation was performed by measuring SNR and CNR values for postcontrast Wave T1 MPRAGE and standard T1 MPRAGE. Signal intensity measurements were performed in ROIs based in the left basal ganglia (gray matter) and in the left inferior frontal subcortical white matter. We sampled noise ROIs measuring 25 voxels in size in air-containing regions above the left aspect of the head for each participant. The standard deviation of the background noise was calculated in these ROIs on the standard T1 MPRAGE and Wave T1 MPRAGE images. The SNR in gray matter and white matter were calculated by dividing the mean signal intensity in the respective ROIs by the standard deviation of the background noise. The contrast to noise ratios were calculated by taking the difference in signal intensities for the gray matter and white matter ROIs and dividing by the standard deviation of the background noise.
Statistical analysis
We tested for noninferiority of Wave-T1-MPRAGE compared to standard T1-MPRAGE in the head-to-head analysis. A noninferiority margin (Δ) of 15% was chosen with the null hypothesis (H0) that the proportion of cases where standard T1-MPRAGE was preferred over Wave-T1-MPRAGE was > 15% [16]. We used the Z statistic to calculate the probability of the standard sequence being preferred over the Wave-T1-MPRAGE sequence in more than 15% of cases (H0 > Δ), with a significance level (α) of 0.05. The required sample size was estimated as described [17] for a single proportion (the proportion of subjects in which visualization of enhancing lesions in all compartments was preferred on standard over Wave-T1-MPRAGE), for a type I error rate (α) of 0.05, a power (1-β) of 0.90 and noninferiority margin of 15%, a minimum of 63 cases was required. Descriptive data were summarized by the calculation of percentile proportions, means and standard deviations. We also calculated the upper bound of the 95% confidence interval for the proportion of cases where standard T1-MPRAGE was preferred over Wave-T1-MPRAGE, i.e., the critical value, pcritical. A Welch’s t-test was used for the comparison of mean SNR/CNR. Interrater agreement was reported using the quadratically weighted Cohen κ to disproportionately penalize larger disagreements. The interrater agreement was interpreted according to Landis and Koch [18]. Intra-rater reliability was measured by intraclass correlation coefficients (ICC) estimates based on a single-measurement, absolute-agreement, two-way mixed-effects model. All statistical calculations were performed using R version 3.6.3.
Results
Of the 96 recruited study participants, 16 were excluded from the comparative evaluation for the following reasons: (a) contrast extravasation during intravenous injection (n = 1), (b) inadvertent mismatches between sequence parameters, including errors in the initial protocol set-up resulting in the acquisition of the Wave sequence with fat-suppression, which restricted the comparison with the standard sequence which did not have fat suppression (n = 10), and (c) errors in the execution of the imaging protocol resulting in the acquisition of only one type of post-contrast T1-weighted sequence during the scan (n = 5). Of note, none of the sequences needed to be repeated because there were two iterations of the same sequence (Wave and standard-T1-MPRAGE) performed after contrast administration. A total of 80 adult participants (mean age, 56 years ± 18 [standard deviation]; 42 men, 38 women) were included in the study. Demographic information for the 80 participants included in the study can be found in Table 2.
The most common clinical indication for MRI examination (Table 2) was the study of neoplastic disease in 64% (51 of 80) of study participants, followed by vascular diseases in 10% (8 of 80), and infection / inflammatory diseases in 2% (2 in 80) of participants. There were other indications for examination of the brain that together accounted for 19 of 80 participants (24%), including headache, trauma and altered mental status. In the head-to-head analysis, abnormal enhancement was detected in 47 of 80 cases (59%). Of the 47 cases that showed abnormal enhancement, 28 (60%) had parenchymal enhancement, 37 (79%) had dural enhancement, 8 (17%) had leptomeningeal enhancement, and 2 (4%) had ependymal enhancement. In 30 of 47 (64%) cases with enhancing lesions, the raters visualized pathological enhancement in more than one category. Fifty-four of 80 studies (67%) were performed with the standard post-contrast T1-MPRAGE sequence acquired before Wave-T1-MPRAGE, and 26 of 80 studies (33%) were performed with the Wave-T1-MPRAGE acquired before the standard T1-MPRAGE sequence.
Wave-T1-MPRAGE was noninferior to standard T1-MPRAGE for delineating enhancement in all 47 cases that showed pathological enhancement. The head-to-head comparison showed that the degree of lesion enhancement of both sequences was indistinguishable in all instances, which enabled the effective characterization of multiple and heterogenous enhancing lesions (Fig. 2) in patients undergoing evaluation for gliomas and metastases. Similarly, the conspicuity of enhancement was also identical for dural lesions and in small metastatic lesions in the posterior fossa (Fig. 3). In Figs. 2 and 3, the Wave sequence was before the standard sequence after administration of contrast.
For the other comparison criteria, interrater agreement ranged from moderate to substantial (κ = 0.59 for motion, p < 0.001; 0.64 for noise, p < 0.001; and 0.74 for the overall diagnostic quality, p < 0.001). Similarly, the ICC for intra-rater reliability ranged from good to excellent (for noise, ICC = 0.83 (S.Y.H.) and 0.80 (C.N.); for motion, ICC = 0.99 (S.Y.H.) and 1 (C.N.); and for diagnostic quality, ICC = 1 for both image reviewers). Wave-T1-MPRAGE was considered noninferior in the perception of noise (upper-bound 95% CI: pcrit = 9%; p < 0.001), motion artifact (upper-bound 95% CI: pcrit = 7%; p < 0.001), and overall diagnostic quality (upper-bound 95% CI: pcrit = 5%; p < 0.001). With respect to image noise, Wave and standard T1-MPRAGE were rated as equivalent in 75 of 80 (94%) cases, the standard sequence was preferred in 4 of 80 (5%) cases, and Wave-T1-MPRAGE was preferred for showing less background noise than the standard sequence in 1 of 80 (1%) cases. In the evaluation of motion, 64 of 80 (80%) cases were considered equivalent. Wave-T1-MPRAGE showed fewer motion artifacts in 13 of 80 (16%) cases, and the standard sequence showed fewer motion artifacts in 3 of 80 (4%) cases. For the overall diagnostic quality, Wave-T1-MPRAGE was preferred in 4 of 80 (5%) of cases, while the standard sequence was preferred in 2 of 80 (2%) of cases. The standard and Wave-T1-MPRAGE sequences were considered equivalent for the overall diagnostic quality in 74 of 80 (93%) of cases. Wave-T1-MPRAGE was also noninferior for the sharpness of images, and both sequences were equally sharp in 78 of 80 (97.5%) of cases. From the total 80 cases, in only one case (1.25%) Wave was considered sharper and in one case the standard sequence was sharper (1.25%). Additional details of the head-to-head comparison and noninferiority testing results are shown in Fig. 4. The quantitative assessment of SNR/CNR demonstrated that Wave-T1-MPRAGE shows significantly reduced mean CNR (p < 0.001), as well as decreased mean SNR in the gray- and white-matter (p < 0.001) compared to the average values in the standard sequence (Fig. 5).
Balloon plot showing the results of the head-to-head comparison of post-contrast standard T1 MPRAGE and post-contrast Wave-CAIPI T1 MPRAGE for visualization of abnormal intracranial enhancing lesions, sharpness, the perception of noise, presence of artifacts due to motion, and the overall diagnostic quality. A zero-score indicates equivalency, negative scores (left) favor standard T1-MPRAGE, and positive scores (right) favor Wave-T1-MPRAGE
Out of the final 80 included participants, 72 individuals (90%) were scanned with a 20-channel coil and 8 individuals (10%) were scanned with a 32-channel coil. Nevertheless, the performance of Wave-T1-MPRAGE did not differ significantly when post-hoc subgroup analyses were performed. Additional details of the head-to-head comparison for each of the two coil set-ups are provided in the Electronic Supplementary Material.
Discussion
This study compared the diagnostic performance of an ultrafast post-contrast 3D Wave-T1-MPRAGE sequence to standard 3D T1-MPRAGE in the evaluation of abnormal intracranial enhancement among patients undergoing routine inpatient and outpatient contrast-enhanced brain MRI at a large volume tertiary-care hospital. The findings show that a Wave-T1-MPRAGE sequence acquired in less than half the time as the standard sequence (2:32 min versus 5:18 min) provides equivalent, robust visualization of all enhancing parenchymal, leptomeningeal and dural lesions with unanimous agreement between expert neuroradiologists. In addition to preserving sharpness (p < 0.001) and overall diagnostic quality (p < 0.001), Wave-T1-MPRAGE was noninferior to standard T1-MPRAGE in the degree of noise (p < 0.001) and motion artifact (p < 0.001).
The use of high-resolution isotropic 3D T1 post-contrast MPRAGE has been established as a workhorse sequence in the systematic evaluation of brain tumors [6], metastatic disease [19], epilepsy [8], and multiple sclerosis [7]. The Wave-CAIPI approach enables higher accelerations than standard parallel imaging with minimal g-factor noise amplification and has been shown to yield comparable diagnostic quality to standard acquisitions, as demonstrated in clinical validation studies of the post-contrast Wave-T1-SPACE sequence for the evaluation of brain metastases [13], Wave susceptibility-weighted imaging for the detection of hemorrhage [10], and non-contrast Wave T1-weighted MPRAGE for the assessment of cortical volumes [11]. The fact that Wave-T1-MPRAGE was rated noninferior with respect to image noise in the current work was interesting given that a signal-to-noise ratio (SNR) reduction of 1/√2 (approximately 30%) is expected due to the higher acceleration factor of the Wave-CAIPI sequence. Despite the reductions in SNR and CNR for the Wave sequences, the qualitative analyses showed that there was no impact on diagnostic quality. This result suggests that the small reduction in SNR of the Wave-T1-MPRAGE sequence did not influence the diagnostic evaluation of the radiologist reviewers. The benefits of our current approach are that the R = 4 images were equivalent to standard MPRAGE for both the 20-ch and 32-ch coils, and were more robust to noise compared to prior studies that used higher acceleration factors with Wave-CAIPI [11]. At higher acceleration factors, the difference in SNR may become more perceptible, which could place a limit on the degree of acceleration that can be clinically achieved. The advantages of using a fast and diagnostically robust acquisition include faster patient turnaround times, which may increase the accessibility and throughput of MRI examinations [20]. The highly accelerated Wave-T1-MPRAGE sequence may particularly benefit acutely ill patients or patients who may be unable to remain still for prolonged periods. In addition, given that the high-resolution 3D T1 post-contrast sequence is usually the last to be performed in contrast-enhanced brain MRI protocols, it is the most susceptible to motion and stands to benefit greatly from faster acquisitions, which have been shown to decrease motion artifact and improving overall image quality [10]. The time savings from Wave-CAIPI sequences are projected to improve patient throughput by minimizing protocol length and improving the operational capacity of imaging services [21]. As a case in point, at our institution, the use of Wave-CAIPI accelerated sequences contributed to streamlining the MRI workflow, which was significantly impacted by the global surge of coronavirus disease 2019 (COVID-19). The changes and restrictions imposed by COVID-19 created a large backlog of imaging studies, which disproportionately affected oncology patients requiring more frequent surveillance examinations [22]. Our findings support the idea that Wave-T1-MPRAGE could replace standard T1-MPRAGE for the clinical evaluation of enhancing brain lesions in both inpatient and outpatient settings and improve patient access to valuable MRI resources. This study has several limitations. First, we sought to balance the order of acquisition to minimize potential differences in conspicuity of enhancement due to the time elapsed from contrast injection. In our cohort, a slight preponderance of studies were acquired with the post-contrast standard T1-MPRAGE (54 of 80, 67%) prior to Wave-T1-MPRAGE, even after inverting the order of sequences halfway through the study, with Wave-T1-MPRAGE acquired prior to the standard sequence in 26 of 80 (33%) cases. Although we did not achieve an even number of each acquisition order, the results showed that the conspicuity of contrast enhancement was rated as equivalent in the entire study sample. The strategy of inverting the order of sequences halfway through the study was mandated by the constraints of staffing and streamlined technologist workflow during the COVID-19 pandemic. In theory, our approach could be subject to bias due to changes in the system, e.g., scanner upgrades and/or degradation, that could affect one order more than the other. In practice, no scanner upgrades occurred during the four-month study period, and system wear and tear was minimal during this time. Alternating the acquisition order in a scan-by-scan fashion is a potential solution that may be implemented in future studies when feasible. Future studies could also focus on characterizing the time-to-peak enhancement of enhancing pathology occurring in different intracranial compartments and timing the acquisition of Wave and standard T1 MPRAGE sequences more precisely in order to ensure that an equal number of cases were acquired with peak enhancement for each sequence. While theoretically more rigorous, we believe this approach is more complex to implement and may not be practically achievable given the variability of enhancement kinetics for different types of intracranial enhancing lesions.
Second, despite being blinded to the acquisition protocol, the reviewers may still have been able to identify images acquired with Wave- versus standard-T1-MPRAGE due to the slightly higher noise at the center of image on the Wave sequence owing to the higher degree of acceleration. An experienced neuroradiologist could become sensitized to the slightly higher noise level after reviewing multiple images, thereby introducing potential bias into the image evaluation. We sought to minimize this possibility by pairing closely the most important parameters that determine image quality and image contrast between acquisitions, including TR, TE, flip angle, and spatial resolution. Lastly, the selection of a proper noninferiority margin for assessing the similarity of diagnostic imaging studies is often challenging. Our choice was guided by a review of similar imaging-based noninferiority publications and consensus among the group of neuroradiologists that the new sequence could be considered noninferior if the standard sequence was preferred in fewer than 15% of cases. Since this threshold is subjective, we also reported the critical value (Pcritical), equivalent to the upper bound of a 95% confidence interval, for the proportion of cases in which the standard sequence was preferred.
In conclusion, contrast-enhanced Wave-CAIPI 3D T1-MPRAGE was noninferior to the standard 3D T1-MPRAGE sequence in the visualization of enhancing lesions and overall diagnostic quality, with an equivalent degree of background noise and susceptibility to motion artifacts. The clinical application of the Wave-T1-MPRAGE enabled a two-fold reduction in the acquisition time of post-contrast images, leading to more efficient utilization of MR resources without loss of clinically important information. At our institution, in accelerating contrast-enhanced brain MRI protocols across a variety of indications, the ultrafast post-contrast Wave-T1-MPRAGE sequence has played an outsize role in improving the diagnostic evaluation and imaging workflow in an inpatient and outpatient setting, with potential for further downstream operational impact and efficiency.
Abbreviations
- 3D:
-
Three-dimensional
- CAIPI:
-
Controlled aliasing in parallel imaging
- CNR:
-
Contrast-to-noise ratio
- GRAPPA:
-
Generalized autocalibrating partially parallel acquisition
- MPRAGE:
-
Magnetization-prepared rapid gradient echo
- SNR:
-
Signal-to-noise ratio
- SPACE:
-
Sampling perfection with application-optimized contrasts using different flip angle evolution
- WL:
-
Window level
- WW:
-
Window width
References
Mugler JP, Brookeman JR (1990) Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 15:152–157. https://doi.org/10.1002/mrm.1910150117
Kato Y, Higano S, Tamura H et al (2009) Usefulness of contrast-enhanced T1-weighted sampling perfection with application-optimized contrasts by using different flip angle evolutions in detection of small brain metastasis at 3 T MR imaging: comparison with magnetization-prepared rapid acquisitio. AJNR Am J Neuroradiol 30:923–929. https://doi.org/10.3174/ajnr.A1506
Jeevanandham B, Kalyanpur T, Gupta P, Cherian M (2017) Comparison of post-contrast 3D-T(1)-MPRAGE, 3D-T(1)-SPACE and 3D-T(2)-FLAIR MR images in evaluation of meningeal abnormalities at 3-T MRI. Br J Radiol 90:20160834. https://doi.org/10.1259/bjr.20160834
Danieli L, Roccatagliata L, Distefano D et al (2022) Nonlesional sources of contrast enhancement on postgadolinium “Black-Blood” 3D T1-SPACE images in patients with multiple sclerosis. AJNR Am J Neuroradiol 43:872–880. https://doi.org/10.3174/ajnr.A7529
Kaufmann TJ, Smits M, Boxerman J et al (2020) Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro Oncol 22:757–772. https://doi.org/10.1093/neuonc/noaa030
Ellingson BM, Bendszus M, Boxerman J et al (2015) Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol 17:1188–1198. https://doi.org/10.1093/neuonc/nov095
Traboulsee A, Simon JH, Stone L et al (2016) Revised recommendations of the consortium of MS centers task force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 37:394–401. https://doi.org/10.3174/ajnr.A4539
Bernasconi A, Cendes F, Theodore WH et al (2019) Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: a consensus report from the International League Against Epilepsy Neuroimaging Task Force. Epilepsia 60:1054–1068. https://doi.org/10.1111/epi.15612
Bilgic B, Gagoski BA, Cauley SF et al (2015) Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med 73:2152–2162. https://doi.org/10.1002/mrm.25347
Conklin J, Longo MGF, Cauley SF et al (2019) Validation of highly accelerated WAVE-CAIPI SWI compared with conventional SWI and T2*-weighted gradient recalled-echo for routine clinical brain MRI at 3 T. AJNR Am J Neuroradiol 40:2073–2080. https://doi.org/10.3174/ajnr.A6295
Longo MGF, Conklin J, Cauley SF et al (2020) Evaluation of ultrafast wave-CAIPI MPRAGE for visual grading and automated measurement of brain tissue volume. AJNR Am J Neuroradiol 41:1388–1396. https://doi.org/10.3174/ajnr.A6703
Ngamsombat C, Filho ALMG, Longo MGF et al (2021) Evaluation of ultrafast Wave-CAIPI 3D FLAIR in the visualization and volumetric estimation of cerebral white matter lesions. AJNR Am J Neuroradiol 42:1584-1590. https://doi.org/10.3174/ajnr.A7191
Goncalves Filho ALM, Conklin J, Longo MGF et al (2020) Accelerated post-contrast wave-CAIPI T1 SPACE achieves equivalent diagnostic performance compared with standard T1 SPACE for the detection of brain metastases in clinical 3-T MRI. Front Neurol 11:587327. https://doi.org/10.3389/fneur.2020.587327
Filho ALMG, LongoMGF, Conklin J et al (2021) MRI Highly Accelerated Wave-CAIPI T1-SPACE versus Standard T1-SPACE to detect brain gadolinium-enhancing lesions at 3 T. J Neuroimaging 31:893-901. https://doi.org/10.1111/jon.12893
Cauley SF, Setsompop K, Bilgic B et al (2017) Autocalibrated wave-CAIPI reconstruction; Joint optimization of k-space trajectory and parallel imaging reconstruction. Magn Reson Med 78:1093–1099. https://doi.org/10.1002/mrm.26499
Ahn S, Park SH, Lee KH (2013) How to demonstrate similarity by using noninferiority and equivalence statistical testing in radiology research. Radiology 267:328–338. https://doi.org/10.1148/radiol.12120725
Chow SC, Wang H, Shao J (2003) Sample size calculations in clinical research. CRC Press, New York
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159. https://doi.org/10.2307/2529310
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Lang M, Cartmell S, Tabari A et al (2021) Evaluation of the Aggregated Time Savings in Adopting Fast Brain MRI Techniques for Outpatient Brain MRI. Acad Radiol S1076-6332(21)00319-6
Boland GW, Duszak R (2015) Modality access: strategies for optimizing throughput. J Am Coll Radiol 12:1073–1075. https://doi.org/10.1016/j.jacr.2015.06.012
Luker GD, Boettcher AN (2021) Impact of COVID-19 on clinical care and research in cancer imaging: where we are now. Radiol Imaging Cancer 3:e210003. https://doi.org/10.1148/rycan.2021210003
Acknowledgements
This work was supported by the National Institutes of Health (grant no. P41EB030006) and a research grant from Siemens Healthineers. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding
This study has received funding from National Institutes of Health (grant no. P41EB030006) and a research grant from Siemens Healthineers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Susie Y. Huang.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Siemens Healthineers.
Statistics and biometry
One of the authors has significant statistical expertise and received advice by the Harvard Catalyst Biostatistical Consulting Program.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• comparative study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 209 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goncalves Filho, A.L.M., Awan, K.M., Conklin, J. et al. Validation of a highly accelerated post-contrast wave-controlled aliasing in parallel imaging (CAIPI) 3D-T1 MPRAGE compared to standard 3D-T1 MPRAGE for detection of intracranial enhancing lesions on 3-T MRI. Eur Radiol 33, 2905–2915 (2023). https://doi.org/10.1007/s00330-022-09265-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09265-6