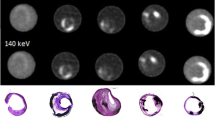
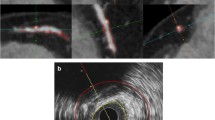
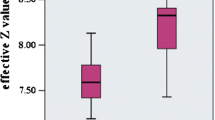
Abstract
Objectives
This study aimed to demonstrate the effectiveness of spectral photon-counting CT (SPCCT) in quantifying fibrous cap (FC) thickness, FC area, and lipid-rich necrotic core (LRNC) area, in excised carotid atherosclerotic plaques by comparing it with histopathological measurements.
Methods
This is a single-center ex vivo cross-sectional observational study. Excised plaques of 20 patients (71 +/- 6 years; 13 men), obtained from carotid endarterectomy were scanned with SPCCT using standardized acquisition settings (120k Vp/19 μA; 7–18 keV, 18–30 keV, 30–45 keV, 45–75 keV, and 75–118 keV). FC thickness, FC area, and LRNC area were quantified and compared between high-resolution 3D multi-energy CT images and histopathology using the Wilcoxon signed-ranks test and Bland–Altman analysis. Images were interpreted twice by two radiologists separately, blinded to the histopathology; inter- and intra-rater reliability were assessed with the intra-class correlation coefficients (ICC).
Results
FC thickness and FC area did not show significant differences between the SPCCT-derived radiological measurements versus the histopathological measurements (p value range 0.15–0.51 for FC thickness and 0.053–0.30 for FC area). For the LRNC area, the p value was statistically non-significant for reader 1 (range 0.36–0.81). The Bland-Altman analysis showed mean difference and 95% confidence interval for FC thickness, FC area, and LRNC area, 0.04 (−0.36 to 0.12) square root mm, −0.18 (−0.34 to −0.02) log10 mm2 and 0.10 (−0.088. to 0.009) log10 mm2 respectively.
Conclusion
The result demonstrated a viable technique for quantifying FC thickness, FC area, and LRNC area due to the combined effect of high spatial and energy resolution of SPCCT.
Key Points
• SPCCT can identify and quantify different components of carotid atherosclerotic plaque in ex vivo study.
• Components of atherosclerotic plaque did not show significant differences between the SPCCT-derived radiological measurements versus the histopathological measurements.




Similar content being viewed by others
Change history
30 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00330-023-09849-w
Abbreviations
- HU:
-
Hounsfield unit
- MD:
-
Material decomposition
- SPCCT:
-
Spectral photon-counting computed tomography
- TIA:
-
Transient ischemic attack
References
World Health Organization (2018) WHO methods and data sources for global burden of disease estimates 2000-2016. Global Health Estimates Technical Paper WHO/HIS/IER/GHE/20184, WHO, Geneva
Ooi YC, Gonzalez NR (2015) Management of extracranial carotid artery disease. Cardiol Clin 33:1–35
Insull W Jr (2009) The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 122:S3–S14
Shah PK (2003) Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol 41:S15–S22
Mughal MM, Khan MK, DeMarco JK, Majid A, Shamoun F, Abela GS (2011) Symptomatic and asymptomatic carotid artery plaque. Expert Rev Cardiovasc Ther 9:1315–1330
Brinjikji W, Huston J, Rabinstein AA, Kim G-M, Lerman A, Lanzino G (2016) Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 124:27–42
Saba L, Saam T, Jäger HR et al (2019) Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 18:559–572
Lin E, Alessio A (2009) What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J Cardiovasc Comput Tomogr 3:403–408
Rothwell PM, Eliasziw M, Gutnikov SA et al (2003) Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 361:107–116
Hellings WE, Peeters W, Moll FL et al (2010) Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 121:1941–1950
Ballabriga R, Alozy J, Campbell M et al (2016) Review of hybrid pixel detector readout ASICs for spectroscopic X-ray imaging. J Instrum 11:P01007
Panta RK, Butler APH, Butler PH et al First human imaging with MARS photon-counting CT2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC). IEEE, pp 1-7
Marfo E, Anderson NG, Butler APH et al (2020) Assessment of material identification errors, image quality and radiation doses using small animal spectral photon-counting CT. IEEE Trans Radiat Plasma Med Sci 5:578–587
Panta RK, Walsh MF, Bell ST, Anderson NG, Butler AP, Butler PH (2014) Energy calibration of the pixels of spectral x-ray detectors. IEEE Trans Med Imaging 34:697–706
Butler PH, Adebileje SA, Alexander SD et al MARS pre-clinical imaging: the benefits of small pixels and good energy dataDevelopments in X-Ray Tomography XII. International Society for Optics and Photonics, pp 111130C–111130C
Aamir R, Chernoglazov A, Bateman CJ et al (2014) MARS spectral molecular imaging of lamb tissue: data collection and image analysis. J Instrum 9:P02005–P02005
Raja AY, Moghiseh M, Bateman CJ et al (2018) Measuring identification and quantification errors in spectral CT material decomposition. Appl Sci 8:467
Bateman CJ, Knight D, Brandwacht B et al (2018) MARS-MD: rejection based image domain material decomposition. J Instrum 13:P05020
Stary HC, Chandler AB, Dinsmore RE et al (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92:1355–1374
Grant EG, Benson CB, Moneta GL et al (2003) Carotid artery stenosis: gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 229:340–346
Stary HC (2000) Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 20:1177–1178
Zainon R, Ronaldson JP, Janmale T et al (2012) Spectral CT of carotid atherosclerotic plaque: comparison with histology. Eur Radiol 22:2581–2588
Cai J, Hatsukami TS, Ferguson MS et al (2005) In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 112:3437–3444
Den Hartog A, Bovens S, Koning W et al (2013) Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 45:7–21
Stephens K (2021) FDA Approves Siemens Healthineers’ Naeotom Alpha CT Scanner. AXIS Imaging News
Koenig T, Hamann E, Procz S et al (2013) Charge summing in spectroscopic x-ray detectors with high-Z sensors. IEEE Trans Nucl Sci 60:4713–4718
Acknowledgements
The authors acknowledge the University of Otago, University of Canterbury, European Council for Nuclear Research (CERN).
Funding
This project was funded by the Ministry of Business, Innovation and Employment (MBIE), New Zealand under contract number UOCX1404, by MARS Bioimaging Ltd, and the Ministry of Education through the MedTech CoRE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
Anthony. P.H. Butler, Professor and head of department of Academic Radiology, University of Otago, Christchurch, New Zealand.
Conflict of interest
Anthony. P.H. Butler is a shareholder and director of MARS Bioimaging Ltd. Shishir Dahal, Steven Gieseg and Aamir Y Raja are shareholders of the company. Others have no financial holding in the company, nor any other conflict of interest to disclose.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
This study is a part of my PhD research. Thus, it has been previously reported in my thesis.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Company name
The scanner used in this research was developed by MARS Bioimaging Ltd., Christchurch, New Zealand.
The work originated from the University of Otago, Christchurch, 2 Riccarton Avenue, Christchurch Central, Christchurch 8011, New Zealand.
The original online version of this article was revised: In this article the affiliation details for Author Aamir Y. Raja were incorrectly given as: 2 Department of Academic Radiology, University of Otago, Christchurch, New Zealand 3 Department of Physics, Khalifa University, Abu Dhabi, UAE. The correct affiliation should have been: 3 Department of Physics, Khalifa University, Abu Dhabi, UAE
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dahal, S., Raja, A.Y., Searle, E. et al. Components of carotid atherosclerotic plaque in spectral photon-counting CT with histopathologic comparison. Eur Radiol 33, 1612–1619 (2023). https://doi.org/10.1007/s00330-022-09155-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09155-x