Abstract
Objectives
Radiomics is a promising avenue in non-invasive characterisation of diffuse glioma. Clinical translation is hampered by lack of reproducibility across centres and difficulty in standardising image intensity in MRI datasets. The study aim was to perform a systematic review of different methods of MRI intensity standardisation prior to radiomic feature extraction.
Methods
MEDLINE, EMBASE, and SCOPUS were searched for articles meeting the following eligibility criteria: MRI radiomic studies where one method of intensity normalisation was compared with another or no normalisation, and original research concerning patients diagnosed with diffuse gliomas. Using PRISMA criteria, data were extracted from short-listed studies including number of patients, MRI sequences, validation status, radiomics software, method of segmentation, and intensity standardisation. QUADAS-2 was used for quality appraisal.
Results
After duplicate removal, 741 results were returned from database and reference searches and, from these, 12 papers were eligible. Due to a lack of common pre-processing and different analyses, a narrative synthesis was sought. Three different intensity standardisation techniques have been studied: histogram matching (5/12), limiting or rescaling signal intensity (8/12), and deep learning (1/12)—only two papers compared different methods. From these studies, histogram matching produced the more reliable features compared to other methods of altering MRI signal intensity.
Conclusion
Multiple methods of intensity standardisation have been described in the literature without clear consensus. Further research that directly compares different methods of intensity standardisation on glioma MRI datasets is required.
Key Points
• Intensity standardisation is a key pre-processing step in the development of robust radiomic signatures to evaluate diffuse glioma.
• A minority of studies compared the impact of two or more methods.
• Further research is required to directly compare multiple methods of MRI intensity standardisation on glioma datasets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult-type diffuse gliomas are a varied group of highly invasive and heterogenous brain tumours (Fig. 1), with an annual US incidence of 5–6/100,000 and glioblastoma (GBM, the most aggressive glioma) accounting for nearly 50% [1]. Despite maximal safe resection of enhancing tumour, and adjuvant therapy with concomitant temozolomide chemotherapy and 60 Grey in 30 fractions of radiotherapy, followed by 6 cycles of temozolomide (‘Stupp protocol’), median overall survival of patients with GBM remains poor at 12–15 months [2, 3].
Multiparametric MRI (mpMRI), with its excellent soft tissue contrast, is frequently used to characterise these tumours [4]. Growing interest in using artificial intelligence (AI) to augment information provided by MRI includes, but is not limited to, non-invasive prediction of cytogenetic alterations, distinguishing treatment effects from pseudoprogression, and distinguishing infiltrative non-enhancing tumour from oedema [5].
Radiomics is a quantitative analytic method of extracting mineable data from medical imaging, and machine learning is typically used to correlate radiomic features and patient-specific data relating to prognosis and/or outcome [6]. Quantitative assessment of the whole tumour volume and surrounding tissues is attractive in the study of a heterogenous disease, which is hampering current treatment strategies [5]. Many radiomic studies evaluating types of diffuse glioma aim to predict prognosis [7], non-invasively diagnose genetic and molecular changes [8] (which play a key role in diagnosis, prognosis, and management), and distinguish between treatment effects and tumour progression [9].
Despite its promise, radiomics has largely been limited to small retrospective proof-of-principle studies, without sufficient evidence to support translation into radiological practice [10]. MRI-based radiomics is limited by the non-biological, scanner-dependent variation in image signal intensity [11,12,13,14]. MR intensity does not map easily to a physical tissue property, in contrast to CT, and shows variation between timepoints, vendors, magnetic field strengths, and acquisition settings [15,16,17,18]. Radiomic features are highly sensitive to the values of the signal intensities in the image, and non-biological alteration must be removed. Therefore, MRI signal intensity must be standardised, i.e. the range and distribution of voxel intensity must be similar across patients, prior to radiomic analysis to ensure that the results are reproducible [11]. Despite this, there is a lack of consensus as to the optimal method when characterising diffuse glioma. Although not a specific diagnosis, diffuse glioma is a useful grouping, as they often share the same radiomics pipeline and are a commonly studied group of related tumours [13, 16]. We aim to perform a systematic review of the literature examining the efficacy of different MRI intensity standardisation procedures prior to the extraction of radiomic features in the setting of adult-type diffuse glioma.
Materials and methods
Search strategy and selection criteria
This systematic review was undertaken according to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis’ (PRISMA) statement. A search of MEDLINE, EMBASE, and SCOPUS databases was performed on 5 October 2021 using the following concepts, linked by the “AND” operator, including synonymous terms that were linked with the “OR” operator: (1) MRI, (2) radiomics, (3) intensity standardisation, and (4) glioma. No limit was placed on the date, language, location, or type of study. Exclusion criteria were the following: non-human based, not regarding adult-type diffuse gliomas, non-original research, non-MR radiomics, no mention of intensity standardisation, or no assessment of the effect of intensity standardisation (compared to another method or to no standardisation). After removing duplicates, articles were screened based on titles and abstract, and subsequently the full text. References in the included articles were manually reviewed. Full search strategy, methodology, and PRISMA checklist are available in the supplementary files.
Quality assessment
Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) was used to assess the risk of bias [19]. QUADAS-2 was used because the objective was to evaluate performance of any given intensity standardisation method, when compared to either no standardisation or another method. QUADAS-2 assesses four domains: (1) patient selection—description of how patients were recruited such as inclusion and exclusion criteria; (2) index test—how the index test was conducted and interpreted; (3) reference standard—how the reference test was conducted and interpreted; and (4) flow and timing—patients that did not have the index or reference test or were excluded from final analysis. Each domain was assessed for risk of bias and the first three domains were also assessed for applicability and categorised as either low risk, high risk, or unclear. The index test was taken to be the intensity standardisation method under investigation, and the reference test was either no standardisation or an alternative method used as a comparator. Two reviewers (F.M., K.F.) independently reviewed each study and any disagreement resolved by consensus.
Results
Search results
After duplicate removal, 741 results were returned from database searches (Fig. 2). Following title and abstract screening, full-text screening was undertaken for 60 articles. Twelve articles meeting the inclusion criteria were included in the review. Two studies by Florez et al [20, 21] were included separately as one used only radiomic features from a fluid-attenuated inversion recovery (FLAIR) sequence [21] and the other used a radiomics extracted from a combination of MRI sequences [20], and this may have an impact upon the results of any intensity standardisation process.
Quality assessment
Risk of bias was assessed for each of the four domains and applicability assessed for the first three domains outlined above. Apart from risk of bias in the patient selection domain and applicability concern for the index test, all other domains were low risk for all studies (Table 1). Ten studies were deemed to have unclear risk due to lack of information on how patients were selected. It was unclear whether institutional patients were selected consecutively or randomly or, if publicly available datasets were used, it was unclear whether any inclusion/exclusion criteria were used to select patients.
For applicability concerns of the index test, two studies [26, 27] were deemed high risk because it was not possible to isolate the effects of standardisation from other pre-processing. Two studies [24, 30] were low risk in all domains. Two studies by Florez et al [20, 21] also included patients with meningioma, but were not thought to be at risk of bias or an applicability concern as the results for the GBM patients were presented separately.
Characteristics of included studies
Significant heterogeneity in the pre-processing steps and in analysis methodology (Table 2) precluded a meta-analysis and a narrative synthesis is presented.
All studies were retrospective, although two studies [24, 30] utilised prospectively acquired data. Eight included multicentre data, and for one [27], it was unclear whether data comprised single or multicentre data. Five studies used a publicly available multicentre dataset from The Cancer Imaging Archive (TCIA) [29], or competition data from the brain tumour image segmentation benchmark (BraTs) [31] in addition to institutional data. One study [27] used only publicly available data.
The aims of the studies can be divided into two groups:
-
1.
To assess the impact of intensity standardisation on the robustness and repeatability of radiomic features, and/or
-
2.
To assess the impact of intensity standardisation on a predictive radiomics model.
Nine studies assessed the impact of intensity standardisation on a predictive model. Five studies assessed the impact of standardisation on feature robustness (two studies included both aims). Three groups, Hoebel et al [30], Carré et al [13], and Orlhac et al [14] used a ‘scan-rescan’ method to test radiomic feature robustness, which involved scanning the same patient after a short interval at different field strengths [13, 14] or on the same machine [30]. Two other studies, Um et al [32] and Reuze et al [26] assessed differences in the feature distribution between paired scanners or the ability of a classifier to distinguish patients scanned internally vs externally [32].
The three main approaches to intensity standardisation can be categorised as histogram matching, deep-learning, or limiting or rescaling the signal intensities. Most of the included studies evaluated one method; however, Carré et al [13] and Hoebel et al [30] used two or more. Further detail on the approaches is discussed in the upcoming sections.
Histogram matching
Histogram matching involves transforming the signal intensities of an image to produce a match between the histogram of the reference and transformed image [25, 33]. The reference histogram is calculated from mean intensities of training images, at pre-specified intensity landmarks [33].
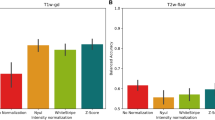
Um et al [32] assessed radiomic feature robustness after the following pre-processing steps: 8-bit rescaling, bias field correction, histogram matching, and isotropic resampling. A Random Forest classifier was used to predict whether images were from internal or external datasets and classification accuracy was measured using the Matthews correlation coefficient. A value of 1 means perfect prediction and 0 no better than chance, and therefore no scanner dependency. The value > 0.2 was taken to mean that images could still retain scanner dependence. Multiple classes of features were extracted. For edge features, different filters (Sobel, Laplacian of Gaussian, Gabor, wavelet) were applied and first-order features extracted. Haralick features were calculated from the grey-level co-occurrence matrices (GLCM). For baseline images, the Matthews correlation coefficients were 0.36, 0.22, and 0.39 (measured from the provided bar chart) for Haralick and the Sobel and Laplacian of Gaussian features, respectively. Histogram matching significantly decreased these to 0.191, 0.170, and 0.140 respectively (p < 0.01).
Zhao et al [34] used histogram specification-grid search (HS-GS), and Chen et al [23] used histogram specification with automated selection of reference frames (HSASR), which automatically select the training histogram. Zhao et al compared the predictive ability of standardised compared to unstandardised images for glioma grading demonstrating an area under the curve (AUC) of 0.956, 27% higher than that without standardisation. Using HSASR, Chen et al achieved 0.9934 AUC for grading (AUC 0.8512 without). These were the highest achieved for glioma grading, although a direct comparison to other methods of intensity standardisation would have been helpful in interpreting the results.
Deep learning
Hu et al [22] describe ‘MIL’ pre-processing and intensity normalisation that corrects: modality incompleteness (M), uneven intensity distribution (I), and inconsistent layer spacing (L) in mpMRI datasets of T1-weighted (T1W), T1Gd, T2-weighted (T2W), and FLAIR sequences. Modality incompleteness is the absence of MRI sequences (referred to as ‘modalities’), for example T1Gd. Intensity unevenness is MRI signal intensity variation, and inconsistent layer spacing refers to variation in slice thickness. Effect of MIL normalisation on accuracy of radiomics model for glioma grading, for isocitrate dehydrogenase 1 (IDH1) prediction (a key genetic marker of adult-type diffuse glioma that has prognostic and diagnostic qualities), and on tumour segmentation was assessed. A cycle-consistent adversarial network (CycleGAN) standardised signal intensities, and a deep learning network synthesised any missing MRI sequences using an encoder (a modified U-net) and separate decoder [22]. Slice thickness was standardised using interpolation software, Statistical Parametric Mapping 12 (SPM12). AUC 0.693 (95% CI 0.613–0.772) was reported for unprocessed images, which increased following synthesis of missing sequences (AUC 0.838, 0.772–0.904), intensity standardisation (0.704, 0.626–0.783), and layer space normalisation (0.716, 0.639–0.793). Combining the three steps produced the best performing model (0.89, 0.838–0.941), highlighting the additive effects of the pre-processing pipeline.
Limiting or rescaling signal intensity
Reuze et al rescaled the signal intensity between 0 and 32767 per patient and concurrently resampled to 0.5 × 0.5 × 0.5 mm3 and assessed the impact on feature robustness on images from 11 MRI scanners [26]. From 31 textural features, 11 were found to be robust among differing magnetic field strength post-normalisation (p > 0.05 on Wilcoxon paired test). Results from intensity standardisation alone were not presented.
Upadhaya et al assessed the effect of pre-processing steps on the accuracy of a overall survival (OS) prediction model [27]. Baseline pre-processing steps included bias field correction, skull stripping, and registration, with additional spatial resampling, intensity quantisation, and normalisation. Intensity normalisation ignored any values outside of the range: (m-s, m+s). m and s are the mean and standard deviation of the intensity values within the VOI. If the model utilised additional sequences and pre-processing steps, sensitivity improved from 79 to 93% and specificity from 86 to 93%. The effect of intensity standardisation alone was not presented.
Florez et al evaluated intensity standardisation on differentiation of tumour volume and oedema in 17 and 20 GBM patients [20, 21]. A 1–99% normalisation, where the 1st and 99th centiles of the intensity histogram are included [28], was compared to no normalisation. Normalised T1Gd sequences produced the best model with an AUC > 0.97 (0.85 without normalisation) [20]. The performance of normalised T2W images decreased—AUC of 0.85 (normalised) compared to AUC 0.91 (without). In a separate study, utilising only FLAIR, normalisation reduced AUC for discriminating tumour and oedema (AUC without 0.87, AUC with normalisation 0.84) [21].
Vils et al assessed the impact of linear intensity interpolation in 118 patients with recurrent GBM [24]. Linear intensity interpolation uses two regions of interests (ROIs) within normal contralateral white matter and the vitreous body:
A radiomic model for prediction of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation (molecular marker for treatment response and prognostication) following normalisation achieved an AUC of 0.673 (95% CI 0.4837–0.8618) on the validation set. Without interpolation, the model achieved an AUC of 0.660 but could not be validated.
Orlhac et al assessed the impact of hybrid WhiteStripe normalisation on the distribution of features from normal white matter and tumours in 18 patients with diffuse glioma that had been scanned and rescanned at different field strengths [14]. WhiteStripe subtracts the mean and divides by the standard deviation of normal white matter intensity [35]. WhiteStripe reduced the number of significantly different features in normal white matter (88 to 69%) and tumour (98 to 60%), highlighting considerable remaining scanner dependency.
Comparison of techniques
Carré et al [13] and Hoebel et al [30] both used histogram-matching and Z-score. Z-score normalisation subtracts the mean signal intensity from each voxel and divides by the standard deviation of the ROI [13]. Carré et al also used WhiteStripe.
Hoebel et al assessed the repeatability, using the intraclass correlation coefficient (ICC), of radiomic features extracted from a set of scan-rescan T1Gd and FLAIR images of 48 patients diagnosed with GBM [30]. Z-score and histogram matching improved repeatability of intensity features on FLAIR but not T1Gd. Histogram matching improved repeatability of texture features on FLAIR (p = 0.003), whereas Z-score did not and neither technique improved the repeatability of texture features on T1Gd.
Carré et al [13] assessed the impact of intensity normalisation on feature robustness and the prediction of glioma grading. Using a scan-rescan dataset of 20 patients with low-grade glioma, histogram matching was found to produce the highest number of robust first-order features on both T1Gd and FLAIR images (ICC and CCC > 0.80, 16 and 8 features out of 18 respectively). Regarding glioma grading using T1Gd images, and only robust features from the first scan-rescan experiment, the average balanced accuracy increased from 0.73 to 0.81, 0.79, and 0.81 for histogram, WhiteStripe, and Z-score respectively.
Discussion
To be clinically useful, radiomics needs to be validated [36], with unique challenges when evaluating radiomic predictive models [37]. For MRI radiomics, a key challenge to assessing repeatability and reproducibility is to remove the scanner-dependent signal intensity changes [11]. This review confirms that intensity standardisation improves radiomic feature repeatability and improves most predictive models, and therefore that the clinical radiologist needs to be aware of this crucial step in any radiomics studies or applications. Variation in methodology precluded the direct comparison of results across studies and this review has highlighted potential areas of improvement, which may improve translation of radiomic models into the clinical setting (Table 3).
In two studies [26, 27], the effects of intensity standardisation were difficult to differentiate from other pre-processing, and the authors could have reported separately the impact of different pre-processing steps on feature robustness or model performance. Hu et al presented all possible combinations of pre-processing steps, with separate AUC results, so the impact of each step was identifiable.
Only two studies [13, 30] compared more than one intensity technique. Given the number of methods and lack of consensus, more studies that directly compare techniques are required. This is important when interpreting the results of histogram specification studies [23, 34]. The AUC for grading was the highest reported; however, it is unclear how this relates to other techniques. A recent analysis [16] compared multiple intensity standardisation techniques and post-feature extraction correction with ComBat, a statistical normalisation for batch-effect correction in genomics that has been applied to radiomics [11, 14]. Intensity standardisation was insufficient to remove scanner dependency, but ComBat could remove scanner-dependent information from extracted features [16], similar to the findings of Orlhac et al [14].
Three studies used scan-rescan data, providing the opportunity to assess radiomic feature reproducibility on images from the same patient acquired within a short time delay (i.e. days between studies). Although a tumour may change microscopically within several days, these radiomic studies assume that if the imaging appearance remains the same then the radiomic features ought to as well [13, 14, 30]. Test-retest data, along with phantom studies [16], and comparison of radiomic features extracted from normal structures provide a useful paradigm to test standardisation techniques. Open access to such data in a public repository should help further validate different intensity standardisation approaches.
Limitations to this review include not being able to retrieve full-text articles for two conference abstracts. Based on the abstracts, it is unlikely they would have been included. Their potential omission will have had a limited impact as a narrative synthesis would still have been required. QUADAS-2 is not specifically designed for assessing the efficacy of MRI intensity standardisation techniques, but we considered this a viable method given the absence of a more specific alternative. The scope of this review was to assess MRI intensity standardisation in the context of diffuse glioma and there will have been the inevitable omission of studies of other organs, brain pathologies, and healthy volunteers.
Conclusion
No clear consensus has emerged as to which approach is the most reliable standardisation approach. In order to translate radiomics to the clinic, studies should assess the effects of intensity standardisation on their results and the impact of any intensity standardisation step should be clearly reported. Collation and sharing of scan-rescan datasets would facilitate production of radiomic models in diffuse glioma and greatly improve the development of clinically translatable models.
Abbreviations
- BraTs:
-
Brain tumour image segmentation benchmark
- CycleGAN:
-
Cycle-consistent adversarial network
- FLAIR:
-
Fluid-attenuated inversion recovery
- GBM:
-
Glioblastoma
- GLCM:
-
Grey-level co-occurrence matrices
- HSASR:
-
Histogram specification with automated selection of reference frames
- HS-GS:
-
Histogram specification-grid search
- ICC:
-
Intraclass correlation coefficient
- IDH1:
-
Isocitrate dehydrogenase 1
- MGMT:
-
- O6-methylguanine-DNA methyltransferase
- mpMRI:
-
Multiparametric MRI
- OS:
-
Overall survival
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies 2
- ROI:
-
Region of interest
- SPM12:
-
Statistical Parametric Mapping 12
- T1Gd:
-
T1-weighted gadolinium enhanced
- T1W:
-
T1-weighted
- T2W:
-
T2-weighted
- TCIA:
-
The Cancer Imaging Archive
- VOI:
-
Volume of interest
References
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS (2018) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol 20:iv1–iv86
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Wen PY, Weller M, Lee EQ et al (2020) Glioblastoma in adults: a Society for Neuro Oncol (SNO) and European Society of Neuro Oncol (EANO) consensus review on current management and future directions. Neuro Oncol 22:1073–1113
Forghani R (2020) Precision digital oncology: emerging role of radiomics-based biomarkers and artificial intelligence for advanced imaging and characterization of brain tumors. Radiol Imaging Cancer 2:e190047
Gillies RJ, Kinahan PE, Hricak H (2015) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Kickingereder P, Burth S, Wick A et al (2016) Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280:880–889
Rathore S, Akbari H, Rozycki M et al (2018) Radiomic MRI signature reveals three distinct subtypes of glioblastoma with different clinical and molecular characteristics, offering prognostic value beyond IDH1. Sci Rep 8:1–12
Akbari H, Rathore S, Bakas S (2018) Quantitative image analysis and machine learning techniques for distinguishing true progression from pseudoprogression in patients with glioblastoma. Neuro Oncol 20:191–192
Pinto dos Santos D, Dietzel M, Baessler B (2021) A decade of radiomics research: are images really data or just patterns in the noise? Eur Radiol 31:2–5
Da-Ano R, Visvikis D, Hatt M (2020) Harmonization strategies for multicenter radiomics investigations. Phys Med Biol 65:24TR02
Yang F, Dogan N, Stoyanova R, Ford JC (2018) Evaluation of radiomic texture feature error due to MRI acquisition and reconstruction: a simulation study utilizing ground truth. Phys Medica 50:26–36
Carré A, Klausner G, Edjlali M et al (2020) Standardization of brain MR images across machines and protocols: bridging the gap for MRI-based radiomics. Sci Rep 10:1–16
Orlhac F, Lecler A, Savatovski J et al (2021) How can we combat multicenter variability in MR radiomics? Validation of a correction procedure. Eur Radiol 31:2272–2280
Traverso A, Wee L, Dekker A, Gillies R (2018) Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys 102:1143–1158
Li Y, Ammari S, Balleyguier C, Lassau N, Chouzenoux E (2021) Impact of preprocessing and harmonization methods on the removal of scanner effects in brain mri radiomic features. Cancers (Basel) 13:1–22
Baeßler B, Weiss K, Dos Santos DP (2019) Robustness and reproducibility of radiomics in magnetic resonance imaging: a phantom study. Investig Radiol 54:221–228
Pandey U, Saini J, Kumar M, Gupta R, Ingalhalikar M (2021) Normative baseline for radiomics in brain MRI: evaluating the robustness, regional variations, and reproducibility on FLAIR images. J Magn Reson Imaging 53:394–407
Whiting PF, Rutjes AWS, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Florez E, Nichols TA, Parker EE, Lirette ST, Howard CM, Fatemi A (2018) Multiparametric magnetic resonance imaging in the assessment of primary brain tumors through radiomic features: a metric for guided radiation treatment planning. Cureus 10:e3426
Florez E, Nichols TA, Lirette ST, Howard CM, Fatemi A (2018) Developing a texture analysis technique using fluid-attenuated inversion recovery (FLAIR) to differentiate tumor from edema for contouring primary intracranial tumors. SM J Clin Med Imaging. 4. 1023.
Hu Z, Zhuang Q, Xiao Y et al (2021) MIL normalisation -- prerequisites for accurate MRI radiomics analysis. Comput Biol Med 133:104403
Chen X, Wu Y, Zhao G et al (2019) Automatic histogram specification for glioma grading using multicenter data. J Healthc Eng 2019:1–12
Vils A, Bogowicz M, Tanadini-Lang S et al (2021) Radiomic analysis to predict outcome in recurrent glioblastoma based on multi-center MR imaging from the Prospective DIRECTOR Trial. Front Oncol 11:636672
Nyúl LG, Udupa JK, Zhang X (2000) New variants of a method of MRI scale standardization. IEEE Trans Med Imaging 19:143–150
Reuzé S, Dirand AS, Sun R et al (2018) A preliminary MRI harmonization method allowing large scale radiomics analysis in glioblastoma. Radiother Oncol 127:S280–S281
Upadhaya T, Morvan Y, Stindel E, Le Reste PJ, Hatt M (2016) Prognosis classification in glioblastoma multiforme using multimodal MRI derived heterogeneity textural features: impact of pre-processing choices. Med Imaging 2016 Comput Diagnosis 9785:97850W
Materka A (2004) Texture analysis methodologies for magnetic resonance imaging. Dialogues Clin Neurosci 6:243–250
Clark K, Vendt B, Smith K et al (2013) The cancer imaging archive (TCIA): maintaining and operating a public information repository. J Digit Imaging 26:1045–1057
Hoebel KV, Patel JB, Beers AL et al (2021) Radiomics repeatability pitfalls in a scan-rescan MRI study of glioblastoma. Radiol Artif Intell 3:e190199
Menze BH, Jakab A, Bauer S et al (2015) The multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans Med Imaging 34:1993–2024
Um H, Tixier F, Bermudez D, Deasy JO, Young RJ, Veeraraghavan H (2019) Impact of image preprocessing on the scanner dependence of multi-parametric MRI radiomic features and covariate shift in multi-institutional glioblastoma datasets. Phys Med Biol 64:165011
Shah M, Xiao Y, Subbanna N et al (2011) Evaluating intensity normalisation on MRIs of human brain with multiple sclerosis. Med Image Anal 15:267–282
Zhao G, Bai J, Wang PP et al (2020) HS–GS: a method for multicenter MR image standardization. IEEE Access 8:158512–158522
Shinohara RT, Shiee N, Reich DS et al (2014) Statistical normalisation techniques for magnetic resonance imaging. Neuroimage Clin 6:9–19
O’Connor JPB, Aboagye EO, Adams JE et al (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14:169–186
Halligan S, Menu Y, Mallett S (2021) Why did European Radiology reject my radiomic biomarker paper? How to correctly evaluate imaging biomarkers in a clinical setting. Eur Radiol 31:9361–9368
Acknowledgements
The authors would like to acknowledge Cancer Research UK funding for the Leeds Radiotherapy Research Centre of Excellence (RadNet; C19942/A28832).
Funding
KF is a 4ward North Clinical PhD fellow funded by Wellcome award 203914/Z/16/Z. Salary for AFS & SC is supported by the Leeds Hospitals Charity and Leeds RadNET, and salary for MGN and AC is supported by RadNET. Salary for JOC is supported by Cancer Research UK Advanced Clinician Scientist Fellowship (C19221/A22746).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is SC.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a systematic review of published literature.
Ethical approval
Institutional Review Board approval was not required because it is a systematic review of published literature.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fatania, K., Mohamud, F., Clark, A. et al. Intensity standardization of MRI prior to radiomic feature extraction for artificial intelligence research in glioma—a systematic review. Eur Radiol 32, 7014–7025 (2022). https://doi.org/10.1007/s00330-022-08807-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08807-2