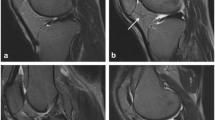
Abstract
Objectives
To investigate the efficacy of fat fraction (FF) and T2* relaxation based on DIXON in the assessment of infrapatellar fat pad (IFP) for knee osteoarthritis (KOA) progression in older adults.
Methods
Ninety volunteers (age range 51–70 years, 65 females) were enrolled in this study. Participants were grouped based on the Kellgren-Lawrence grading (KLG). The FF and T2* values were measured based on the 3D-modified DXION technique. Cartilage defects, bone marrow lesions, and synovitis were assessed based on a modified version of whole-organ magnetic resonance imaging score (WORMS). Knee pain was assessed by self-administered Western Ontario and McMaster Osteoarthritis Index (WOMAC) questionnaire. The differences of FF and T2* measurement and the correlation with WORMS and WOMAC assessments were analyzed. Diagnostic efficiency was analyzed by using receiver operating characteristic (ROC) curves.
Results
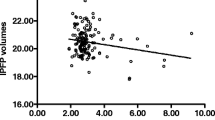
A total of 60 knees were finally included (n = 20 in each group). The values were 82.6 ± 3.7%, 74.7 ± 5.4%, and 60.5 ± 14.1% for FF is the no OA, mild OA, and advanced OA groups, and were 50.7 ± 6.6 ms, 44.1 ± 6.6 ms, and 39.1 ± 4.2 ms for T2*, respectively (all p values < 0.001). The WORMS assessment and WOMAC pain assessment showed negative correlation with FF and T2* values. The ROC showed the area under the curve (AUC), sensitivity, and specificity for diagnosing OA were 0.93, 77.5%, and 100% using FF, and were 0.86, 75.0%, and 90.0% using T2*, respectively.
Conclusions
FF and T2* alternations in IFP are associated with knee structural abnormalities and clinical symptoms cross-sectionally and may have the potential to predict the severity of KOA.
Key Points
• Fat fraction (FF) and T2* relaxation based on DIXON imaging are novel methods to quantitatively assess the infrapatellar fat pad for knee osteoarthritis (KOA) progression in older adults.
• The alterations of FF and T2* using mDIXON technique in IFP were associated with knee structural abnormalities and clinical symptoms.
• FF and T2* alternations in IFP can serve as the new imaging biomarkers for fast, simple, and noninvasive assessment in KOA.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CE-MRI:
-
Contrast-enhanced magnetic resonance imaging
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- FF:
-
Fat fraction
- ICC:
-
Intraclass correlation coefficient
- IFP:
-
Infrapatellar fat pad
- IS:
-
Signal intensity
- JSN:
-
Toint space narrowing
- KLG:
-
Kellgren-Lawrence grading
- KOA:
-
Knee osteoarthritis
- PDW-SPAIR:
-
Proton density-weighted spectrally adiabatic inversion recovery
- ROC:
-
Receiver operating characteristic
- ROI:
-
Global region-of-interest
- T1WI:
-
T1-weighted image
- WOMAC:
-
Western Ontario and McMaster Osteoarthritis Index
- WORMS:
-
Whole-organ magnetic resonance imaging score
References
Emmert D, Rasche T, Stieber C, Conrad R, Mücke M (2018) Knee pain - symptoms, diagnosis and therapy of osteoarthritis. MMW Fortschr Med 160:58–64
Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA (2017) Basic science of articular cartilage. Clin Sports Med 36:413–425
Dobson GP, Letson HL, Grant A et al (2018) Defining the osteoarthritis patient: back to the future. Osteoarthritis Cartilage 26:1003–1007
Ioan-Facsinay A, Kloppenburg M (2013) An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther 15:225
Zeng N, Yan ZP, Chen XY, Ni GX (2020) Infrapatellar fat pad and knee osteoarthritis. Aging Dis 11:1317–1328
Poole AR (2012) Osteoarthritis as a whole joint disease. HSS J 8:4–6
Steidle-Kloc E, Culvenor AG, Dörrenberg J, Wirth W, Ruhdorfer A, Eckstein F (2018) Relationship between knee pain and infrapatellar fat pad morphology: a within- and between-person analysis from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 70:550–557
Teichtahl AJ, Wulidasari E, Brady SR et al (2015) A large infrapatellar fat pad protects against knee pain and lateral tibial cartilage volume loss. Arthritis Res Ther 17:318
Ruan G, Xu J, Wang K et al (2018) Associations between knee structural measures, circulating inflammatory factors and MMP13 in patients with knee osteoarthritis. Osteoarthritis Cartilage 26:1063–1069
Favero M, El-Hadi H, Belluzzi E et al (2017) Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology (Oxford) 56:1784–1793
Mathiessen A, Conaghan PG (2017) Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 19:18
Sae-Jung T, Leearamwat N, Chaiseema N et al (2021) The infrapatellar fat pad produces interleukin-6-secreting T cells in response to a proteoglycan aggrecan peptide and provides dominant soluble mediators different from that present in synovial fluid. Int J Rheum Dis 24:834–846
Eymard F, Pigenet A, Citadelle D et al (2014) Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol 66:2165–2174
Chang J, Liao Z, Lu M, Meng T, Han W, Ding C (2018) Systemic and local adipose tissue in knee osteoarthritis. Osteoarthritis Cartilage 26:864–871
An JS, Tsuji K, Onuma H et al (2021) Inhibition of fibrotic changes in infrapatellar fat pad alleviates persistent pain and articular cartilage degeneration in monoiodoacetic acid-induced rat arthritis model. Osteoarthritis Cartilage 29:380–388
van der Heijden RA, de Vries BA, Poot DHJ et al (2021) Quantitative volume and dynamic contrast-enhanced MRI derived perfusion of the infrapatellar fat pad in patellofemoral pain. Quant Imaging Med Surg 11:133–142
de Vries BA, van der Heijden RA, Poot DHJ et al (2020) Quantitative DCE-MRI demonstrates increased blood perfusion in Hoffa’s fat pad signal abnormalities in knee osteoarthritis, but not in patellofemoral pain. Eur Radiol 30:3401–3408
Crema MD, Felson DT, Roemer FW et al (2013) Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced MRI and association with pain. The MOST study. Osteoarthritis Cartilage 21:413–418
Wang K, Ding C, Hannon MJ, Chen Z, Kwoh CK, Hunter DJ (2019) Quantitative signal intensity alteration in infrapatellar fat pad predicts incident radiographic osteoarthritis: the osteoarthritis initiative. Arthritis Care Res (Hoboken) 71:30–38
Han W, Aitken D, Zheng S et al (2019) Association between quantitatively measured infrapatellar fat pad high signal-intensity alteration and magnetic resonance imaging-assessed progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 71:638–646
Lu M, Chen Z, Han W et al (2016) A novel method for assessing signal intensity within infrapatellar fat pad on MR images in patients with knee osteoarthritis. Osteoarthritis Cartilage 24:1883–1889
Han W, Aitken D, Zhu Z et al (2016) Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis 75:1783–1788
Pezeshk P, Alian A, Chhabra A (2017) Role of chemical shift and Dixon based techniques in musculoskeletal MR imaging. Eur J Radiol 94:93–100
Kim D, Kim SK, Lee SJ, Choo HJ, Park JW, Kim KY (2019) Simultaneous estimation of the fat fraction and R2(*) via T2(*)-corrected 6-echo Dixon volumetric interpolated breath-hold examination imaging for osteopenia and osteoporosis detection: correlations with sex, age, and menopause. Korean J Radiol 20:916–930
Netaji A, Jain V, Gupta AK, Kumar U, Jana M (2020) Utility of MR proton density fat fraction and its correlation with ultrasonography and biochemical markers in nonalcoholic fatty liver disease in overweight adolescents. J Pediatr Endocrinol Metab 33:473–479
Guo Y, Chen Y, Zhang X et al (2019) Magnetic susceptibility and fat content in the lumbar spine of postmenopausal women with varying bone mineral density. J Magn Reson Imaging 49:1020–1028
Cunha GM, Thai TT, Hamilton G et al (2020) Accuracy of common proton density fat fraction thresholds for magnitude- and complex-based chemical shift-encoded MRI for assessing hepatic steatosis in patients with obesity. Abdom Radiol (NY) 45:661–671
Obmann VC, Marx C, Berzigotti A et al (2019) Liver MRI susceptibility-weighted imaging (SWI) compared to T2* mapping in the presence of steatosis and fibrosis. Eur J Radiol 118:66–74
Tao H, Qiao Y, Hu Y et al (2018) Quantitative T2-mapping and T2(⁎)-mapping evaluation of changes in cartilage matrix after acute anterior cruciate ligament rupture and the correlation between the results of both methods. Biomed Res Int 2018:7985672
Altman RD, Gold GE (2007) Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 15 Suppl a:A1-56.
Pietrosimone B, Luc BA, Duncan A, Saliba SA, Hart JM, Ingersoll CD (2017) Association between the single assessment numeric evaluation and the Western Ontario and McMaster universities osteoarthritis index. J Athl Train 52:526–533
Bainbridge A, Bray TJP, Sengupta R, Hall-Craggs MA (2020) Practical approaches to bone marrow fat fraction quantification across magnetic resonance imaging platforms. J Magn Reson Imaging 52:298–306
Peterfy CG, Guermazi A, Zaim S et al (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190
Fontanella CG, Belluzzi E, Rossato M et al (2019) Quantitative MRI analysis of infrapatellar and suprapatellar fat pads in normal controls, moderate and end-stage osteoarthritis. Ann Anat 221:108–114
Reeder SB, Bice EK, Yu H, Hernando D, Pineda AR (2012) On the performance of T2* correction methods for quantification of hepatic fat content. Magn Reson Med 67:389–404
Fukuzawa K, Hayashi T, Takahashi J et al (2017) Evaluation of six-point modified Dixon and magnetic resonance spectroscopy for fat quantification: a fat-water-iron phantom study. Radiol Phys Technol 10:349–358
Acknowledgements
We would like to thank Professor Yanqiu Feng from Southern Medical University for his technical support and all necessary help to the research institute.
Funding
This study has received funding by the National Natural Science Foundation of China (81801653), and General project of President’s Foundation of the Third Affiliated Hospital of Southern Medical University (YM2021012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yanqiu, Feng, the Southern Medical University, and his e-mail address is foree@163.com.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, X., Li, M. et al. Quantitative MR evaluation of the infrapatellar fat pad for knee osteoarthritis: using proton density fat fraction and T2* relaxation based on DIXON. Eur Radiol 32, 4718–4727 (2022). https://doi.org/10.1007/s00330-022-08561-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08561-5