Abstract
Objectives
The purpose of this study was to investigate whether pretreatment kinetic features from ultrafast DCE-MRI are associated with pathological complete response (pCR) in patients with invasive breast cancer and according to immunohistochemistry (IHC) subtype.
Methods
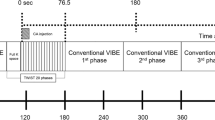
Between August 2018 and June 2019, 256 consecutive breast cancer patients (mean age, 50.2 years; range, 25–86 years) who underwent both ultrafast and conventional DCE-MRI and surgery following neoadjuvant chemotherapy were included. DCE-MRI kinetic features were obtained from pretreatment MRI data. Time-to-enhancement, maximal slope (MS), and volumes at U1 and U2 (U1, time point at which the lesion starts to enhance; U2, subsequent time point after U1) were derived from ultrafast MRI. Logistic regression analysis was performed to identify factors associated with pCR.
Results
Overall, 41.4% of all patients achieved pCR. None of the kinetic features was associated with pCR when including all cancers. Among ultrafast DCE-MRI kinetic features, a lower MS (OR, 0.982; p = 0.040) was associated with pCR at univariable analysis in hormone receptor (HR)–positive cancers. In triple-negative cancers, a higher volume ratio U1/U2 was associated with pCR at univariable (OR, 11.787; p = 0.006) and multivariable analysis (OR, 14.811; p = 0.005). Among conventional DCE-MRI kinetic features, a lower peak enhancement (OR, 0.993; p = 0.031) and a lower percentage of washout (OR, 0.904; p = 0.039) was associated with pCR only in HR-positive cancers at univariable analysis.
Conclusions
A higher volume ratio of U1/U2 derived from ultrafast DCE-MRI was independently associated with pCR in triple-negative invasive breast cancer.
Key Points
• The ratio of tumor volumes obtained at the first (U1) and second time points (U2) of enhancement was independently associated with pCR in triple-negative invasive breast cancers.
• Ultrafast MRI has the potential to improve accuracy in predicting treatment response and personalizing therapy.



Similar content being viewed by others
Abbreviations
- CAD:
-
Computer-aided diagnosis
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- FOV:
-
Field of view
- HER2:
-
Human epidermal growth factor receptor type 2
- HR:
-
Hormone receptor
- ICC:
-
Intraclass correlation coefficient
- IHC:
-
Immunohistochemistry
- MS:
-
Maximal slope
- NAC:
-
Neoadjuvant chemotherapy
- OR:
-
Odds ratio
- pCR:
-
Pathological complete response
- PR:
-
Progesterone receptor
- TTE:
-
Time-to-enhancement
References
Korde LA, Somerfield MR, Carey LA et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39:1485–1505
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292:520–536
Scheel JR, Kim E, Partridge SC et al (2018) MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 Trial. AJR Am J Roentgenol 210:1376–1385
Hylton NM, Blume JD, Bernreuter WK et al (2012) Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology 263:663–672
Gao Y, Heller SL (2020) Abbreviated and ultrafast breast MRI in clinical practice. Radiographics 40:1507–1527
Herrmann KH, Baltzer PA, Dietzel M et al (2011) Resolving arterial phase and temporal enhancement characteristics in DCE MRM at high spatial resolution with TWIST acquisition. J Magn Reson Imaging 34:973–982
Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B (2014) A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol 49:579–585
Mus RD, Borelli C, Bult P et al (2017) Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol 89:90–96
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Haque W, Verma V, Hatch S, Klimberg VS, Butler BE, Teh BS (2018) Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat 170:559–567
Morris EA, Comstock CE, Lee CH et al (2013) ACR BI-RADS® magnetic resonance imaging In: ACR BI-RADS® atlas, breast imaging reporting and data system. American College of Radiolgy, Reston, VA
Honda M, Kataoka M, Onishi N et al (2020) New parameters of ultrafast dynamic contrast-enhanced breast MRI using compressed sensing. J Magn Reson Imaging 51:164–174
van Roozendaal LM, de Wilt JH, van Dalen T et al (2015) The value of completion axillary treatment in sentinel node positive breast cancer patients undergoing a mastectomy: a Dutch randomized controlled multicentre trial (BOOG 2013–07). BMC Cancer 15:610
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Song SE, Cho KR, Seo BK, Woo OH, Jung SP, Sung DJ (2019) Kinetic features of invasive breast cancers on computer-aided diagnosis using 3T MRI data: correlation with clinical and pathologic prognostic factors. Korean J Radiol 20:411–421
Baron P, Beitsch P, Boselli D et al (2016) Impact of tumor size on probability of pathologic complete response after neoadjuvant chemotherapy. Ann Surg Oncol 23:1522–1529
Resende U, Cabello C, Ramalho SOB, Zeferino LC (2019) Prognostic assessment of breast carcinoma submitted to neoadjuvant chemotherapy with pathological non-complete response. BMC Cancer 19:601
Onishi N, Sadinski M, Hughes MC et al (2020) Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer. Breast Cancer Res 22:58
Gajdos C, Tartter PI, Estabrook A, Gistrak MA, Jaffer S, Bleiweiss IJ (2002) Relationship of clinical and pathologic response to neoadjuvant chemotherapy and outcome of locally advanced breast cancer. J Surg Oncol 80:4–11
Bonadonna G, Veronesi U, Brambilla C et al (1990) Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst 82:1539–1545
Goorts B, van Nijnatten TJ, de Munck L et al (2017) Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat 163:83–91
Kim SY, Cho N, Choi Y et al (2020) Ultrafast dynamic contrast-enhanced breast MRI: lesion conspicuity and size assessment according to background parenchymal enhancement. Korean J Radiol 21:561–571
Shin SU, Cho N, Kim SY, Lee SH, Chang JM, Moon WK (2020) Time-to-enhancement at ultrafast breast DCE-MRI: potential imaging biomarker of tumour aggressiveness. Eur Radiol 30:4058–4068
Goto M, Sakai K, Yokota H et al (2019) Diagnostic performance of initial enhancement analysis using ultra-fast dynamic contrast-enhanced MRI for breast lesions. Eur Radiol 29:1164–1174
Goh CW, Wu J, Ding S et al (2019) Invasive ductal carcinoma with coexisting ductal carcinoma in situ (IDC/DCIS) versus pure invasive ductal carcinoma (IDC): a comparison of clinicopathological characteristics, molecular subtypes, and clinical outcomes. J Cancer Res Clin Oncol 145:1877–1886
Kato E, Mori N, Mugikura S, Sato S, Ishida T, Takase K (2021) Value of ultrafast and standard dynamic contrast-enhanced magnetic resonance imaging in the evaluation of the presence and extension of residual disease after neoadjuvant chemotherapy in breast cancer. Jpn J Radiol 39:791–801
Yamaguchi A, Honda M, Ishiguro H et al (2021) Kinetic information from dynamic contrast-enhanced MRI enables prediction of residual cancer burden and prognosis in triple-negative breast cancer: a retrospective study. Sci Rep 11:10112
Song SE, Seo BK, Cho KR et al (2020) Preoperative tumor size measurement in breast cancer patients: which threshold is appropriate on computer-aided detection for breast MRI? Cancer Imaging 20:32
Funding
This study has received funding by a faculty research grant of Yonsei University College of Medicine for 2019 (6–2019-0178) and a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03035995).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Vivian Youngjean Park, MD, PhD, Assistant professor of the Department of Radiology, Severance Hospital, Yonsei University, College of Medicine.
Conflict of Interest
The authors declare no competing interests.
Statistics and Biometry
One of the authors (Hye Jung Shin) has significant statistical expertise.
Informed Consent
Written informed consent was waived by the Institutional Review Board.
Ethical Approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective.
• Observational.
• Performed at one institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, J.H., Park, V.Y., Shin, H.J. et al. Ultrafast dynamic contrast-enhanced breast MRI: association with pathologic complete response in neoadjuvant treatment of breast cancer. Eur Radiol 32, 4823–4833 (2022). https://doi.org/10.1007/s00330-021-08530-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08530-4