Abstract
Objectives
To determine if golden-angle radial sparse parallel (GRASP) dynamic contrast-enhanced (DCE)-MRI allows simultaneous evaluation of perfusion and morphology in liver fibrosis.
Methods
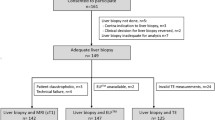
Participants who were scheduled for liver biopsy or resection were enrolled (NCT02480972). Images were reconstructed at 12-s temporal resolution for morphologic assessment and at 3.3-s temporal resolution for quantitative evaluation. The image quality of the morphologic images was assessed on a four-point scale, and the Liver Imaging Reporting and Data System score was recorded for hepatic observations. Comparisons were made between quantitative parameters of DCE-MRI for the different fibrosis stages, and for hepatocellular carcinoma (HCCs) with different LR features.
Results
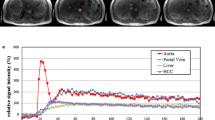
DCE-MRI of 64 participants (male = 48) were analyzed. The overall image quality consistently stood at 3.5 ± 0.4 to 3.7 ± 0.4 throughout the exam. Portal blood flow significantly decreased in participants with F2–F3 (n = 18, 175 ± 110 mL/100 mL/min) and F4 (n = 12, 98 ± 47 mL/100 mL/min) compared with those in participants with F0–F1 (n = 34, 283 ± 178 mL/100 mL/min, p < 0.05 for all). In participants with F4, the arterial fraction and extracellular volume were significantly higher than those in participants with F0–F1 and F2–F3 (p < 0.05). Compared with HCCs showing non-LR-M features (n = 16), HCCs with LR-M (n = 5) had a significantly prolonged mean transit time and lower arterial blood flow (p < 0.05).
Conclusions
Liver MRI using GRASP obtains both sufficient spatial resolution for confident diagnosis and high temporal resolution for pharmacokinetic modeling. Significant differences were found between the MRI-derived portal blood flow at different hepatic fibrosis stages.
Key Points
-
A single MRI examination is able to provide both images with sufficient spatial resolution for anatomic evaluation and those with high temporal resolution for pharmacokinetic modeling.
-
Portal blood flow was significantly lower in clinically significant hepatic fibrosis and mean transit time and extracellular volume increased in cirrhosis, compared with those in no or mild hepatic fibrosis.
-
HCCs with different LR features showed different quantitative parameters of DCE-MRI: longer mean transit time and lower arterial flow were observed in HCCs with LR-M features.





Similar content being viewed by others
Abbreviations
- AF:
-
Arterial fraction
- AIF:
-
Arterial input function
- AUC:
-
Area under the curve
- DCE:
-
Dynamic contrast-enhanced
- ECV:
-
Extracellular volume
- GRASP:
-
Golden-angle radial sparse parallel
- HCC:
-
Hepatocellular carcinoma
- ICC:
-
Intraclass correlation coefficient
- LI-RADS:
-
Liver Imaging Reporting and Data System
- LOA:
-
Limit of agreement
- MRI:
-
Magnetic resonance imaging
- MTT:
-
Mean transit time
- T1WI:
-
T1-weighted image
- VIF:
-
Venous input function
References
Shin GW, Zhang Y, Kim MJ et al (2018) Role of dynamic contrast-enhanced MRI in evaluating the association between contralateral parenchymal enhancement and survival outcome in ER-positive, HER2-negative, node-negative invasive breast cancer. J Magn Reson Imaging 48:1678–1689
Li KL, Lewis D, Jackson A, Zhao S, Zhu X (2018) Low-dose T1W DCE-MRI for early time points perfusion measurement in patients with intracranial tumors: A pilot study applying the microsphere model to measure absolute cerebral blood flow. J Magn Reson Imaging 48:543–557
Cristel G, Esposito A, Damascelli A et al (2019) Can DCE-MRI reduce the number of PI-RADS v.2 false positive findings? Role of quantitative pharmacokinetic parameters in prostate lesions characterization. Eur J Radiol 118:51–57
Hagiwara M, Rusinek H, Lee VS et al (2008) Advanced liver fibrosis: diagnosis with 3D whole-liver perfusion mr imaging—initial experience1. Radiology 246:926–934
San Koh T, Thng CH, Lee PS et al (2008) Hepatic metastases: in vivo assessment of perfusion parameters at dynamic contrast-enhanced MR imaging with dual-input two-compartment tracer kinetics model1. Radiology 249:307–320
Pandharipande PV, Krinsky GA, Rusinek H, Lee VS (2005) Perfusion imaging of the liver: current challenges and future goals. Radiology 234:661–673
Winkel DJ, Heye TJ, Benz MR et al (2019) Compressed sensing radial sampling MRI of prostate perfusion: utility for detection of prostate cancer. Radiology 290:702–708
Gill AB, Black RT, Bowden DJ, Priest AN, Graves MJ, Lomas DJ (2014) An investigation into the effects of temporal resolution on hepatic dynamic contrast-enhanced MRI in volunteers and in patients with hepatocellular carcinoma. Phys Med Biol 59:3187–3200
Chandarana H, Feng L, Ream J et al (2015) Respiratory motion-resolved compressed sensing reconstruction of free-breathing radial acquisition for dynamic liver magnetic resonance imaging. Invest Radiol 50:749–756
Chandarana H, Feng L, Block TK et al (2013) Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol 48:10–16
Yoon JH, Lee JM, Yu MH et al (2018) Evaluation of transient motion during gadoxetic acid-enhanced multiphasic liver magnetic resonance imaging using free-breathing golden-angle radial sparse parallel magnetic resonance imaging. Invest Radiol 53:52–61
Chernyak V, Fowler KJ, Kamaya A et al (2018) Liver Imaging Reporting and Data System (LI-RADS) Version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289:816–830
Sourbron S, Sommer WH, Reiser MF, Zech CJ (2012) Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology 263:874–883
Juluru K, Talal AH, Yantiss RK et al (2017) Diagnostic accuracy of intracellular uptake rates calculated using dynamic Gd-EOB-DTPA-enhanced MRI for hepatic fibrosis stage. J Magn Reson Imaging 45:1177–1185
Armbruster M, Sourbron S, Haug A et al (2014) Evaluation of neuroendocrine liver metastases: a comparison of dynamic contrast-enhanced magnetic resonance imaging and positron emission tomography/computed tomography. Invest Radiol 49:7–14
Stanisz GJ, Odrobina EE, Pun J et al (2005) T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med 54:507–512
Lu H, Clingman C, Golay X, van Zijl PC (2004) Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 52:679–682
Park YN, Chon CY, Park JB et al (1999) Histological grading and staging of chronic hepatitis standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Diseases. Korean J Pathol 33:337–346
Bosman FTFC, Hruban RH, Theise ND (2010) WHO classification of tumours. Digestive System Tumours, 5th edn. World Health Organization, Geneva, Switzerland
Ueno Y, Maeda T, Tanaka U et al (2016) Evaluation of interobserver variability and diagnostic performance of developed MRI-based radiological scoring system for invasive placenta previa. J Magn Reson Imaging 44:573–583
Groszmann R, Kotelanski B, Cohn JN, Khatri IM (1972) Quantitation of portasystemic shunting from the splenic and mesenteric beds in alcoholic liver disease. Am J Med 53:715–722
Kotelanski B, Groszmann R, Cohn JN (1972) Circulation times in the splanchnic and hepatic beds in alcoholic liver disease. Gastroenterology 63:102–111
Iredale JP (2007) Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117:539–548
Berzigotti A, Bosch J (2014) Pharmacologic management of portal hypertension. Clin Liver Dis 18:303–317
Greenway CV, Lawson AE, Stark RD (1967) The effect of haemorrhage on hepatic artery and portal vein flows in the anesthetized cat. J Physiol 193:375–379
Poisson J, Lemoinne S, Boulanger C et al (2017) Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol 66:212–227
Bosch J, Garcia-Pagan JC (2000) Complications of cirrhosis I. Portal hypertension. J Hepatol 32:141–156
Lee DH, Ahn JH, Chung JW et al (2019) Varices on computed tomography are surrogate of clinically significant portal hypertension and can predict survival in compensated cirrhosis patients. J Gastroenterol Hepatol 34:450–457
Iwakiri Y (2014) Pathophysiology of portal hypertension. Clin Liver Dis 18:281–291
Okada M, Kim T, Murakami T (2011) Hepatocellular nodules in liver cirrhosis: state of the art CT evaluation (perfusion CT/volume helical shuttle scan/dual-energy CT, etc.). Abdom Imaging 36:273–281
Weiss J, Ruff C, Grosse U et al (2019) Assessment of hepatic perfusion using GRASP MRI: bringing liver MRI on a new level. Invest Radiol 54:737–743
Choi SH, Lee SS, Park SH et al (2019) LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology 290:388–397
Leporq B, Dumortier J, Pilleul F, Beuf O (2012) 3D-liver perfusion MRI with the MS-325 blood pool agent: a noninvasive protocol to asses liver fibrosis. J Magn Reson Imaging 35:1380–1387
Ou HY, Bonekamp S, Bonekamp D et al (2013) MRI arterial enhancement fraction in hepatic fibrosis and cirrhosis. AJR Am J Roentgenol 201:W596–W602
Bradley SE (1949) Variations in hepatic blood flow in man during health and disease. N Engl J Med 240:456–461
Feng AC, Fan HL, Chen TW, Hsieh CB (2014) Hepatic hemodynamic changes during liver transplantation: a review. World J Gastroenterol 20:11131–11141
Parikh N, Ream JM, Zhang HC, Block KT, Chandarana H, Rosenkrantz AB (2016) Performance of simultaneous high temporal resolution quantitative perfusion imaging of bladder tumors and conventional multi-phase urography using a novel free-breathing continuously acquired radial compressed-sensing MRI sequence. Magn Reson Imaging 34:694–698
Eikefjord E, Andersen E, Hodneland E et al (2015) Use of 3D DCE-MRI for the estimation of renal perfusion and glomerular filtration rate: an intrasubject comparison of FLASH and KWIC with a comprehensive framework for evaluation. AJR Am J Roentgenol 204:W273–W281
Wake N, Chandarana H, Rusinek H et al (2018) Accuracy and precision of quantitative DCE-MRI parameters: how should one estimate contrast concentration? Magn Reson Imaging 52:16–23
Mansour R, Thibodeau Antonacci A, Bilodeau L et al (2020) Impact of temporal resolution and motion correction for dynamic contrast-enhanced MRI of the liver using an accelerated golden-angle radial sequence. Phys Med Biol 65:085004
Feng L, Wen Q, Huang C, Tong A, Liu F, Chandarana H (2020) GRASP-Pro: imProving GRASP DCE-MRI through self-calibrating subspace-modeling and contrast phase automation. Magn Reson Med 83:94–108
Hectors SJ, Lewis S, Kennedy P et al (2020) Assessment of hepatocellular carcinoma response to (90)Y radioembolization using dynamic contrast material-enhanced MRI and intravoxel incoherent motion diffusion-weighted imaging. Radiol Imaging Cancer 2:e190094
Ghodasara S, Chen Y, Pahwa S et al (2020) Quantifying perfusion properties with DCE-MRI using a dictionary matching approach. Sci Rep 10:10210
Acknowledgements
We thank Benjamin Latimer, BA, for his editorial assistance.
Funding
This study was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2013R1A1A2A10066037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Jeong Min Lee.
Conflict of interest
Two authors (R. Grimm and Y. Son) are employees of Siemens Healthineers. Otherwise, the authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were used for this paper.
Informed consent
Written informed consent was obtained from all participants.
Ethical approval
Approval from the Institutional Review Board of Seoul National University Hospital was obtained.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 448 kb)
Rights and permissions
About this article
Cite this article
Yoon, J.H., Lee, J.M., Yu, M.H. et al. Simultaneous evaluation of perfusion and morphology using GRASP MRI in hepatic fibrosis. Eur Radiol 32, 34–45 (2022). https://doi.org/10.1007/s00330-021-08087-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08087-2