Abstract
Objective
The non-invasive discrimination of significant fibrosis (≥ F2) in patients with chronic liver disease (CLD) is clinically critical but technically challenging. We aimed to develop an updated deep learning radiomics model of elastography (DLRE2.0) based on our previous DLRE model to achieve significantly improved performance in ≥ F2 evaluation.
Methods
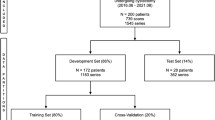
This was a retrospective multicenter study with 807 CLD patients and 4842 images from three hospitals. All of these patients have liver biopsy results as referenced standard. Multichannel deep learning radiomics models were developed. Elastography images, gray-scale images of the liver capsule, gray-scale images of the liver parenchyma, and serological results were gradually integrated to establish different diagnosis models, and the optimal model was selected for assessing ≥ F2. Its accuracy was thoroughly investigated by applying different F0–1 prevalence cohorts and independent external test cohorts. Analysis of receiver operating characteristic (ROC) curves was performed to calculate the area under the ROC curve (AUC) for significance of fibrosis (≥ F2) and cirrhosis (F4).
Results
The AUC of the DLRE2.0 model significantly increased to 0.91 compared with the DLRE model (AUC 0.83) when evaluating ≥ F2 (p = 0.0167). However, it did not show statistically significant differences as integrating gray-scale images and serological data into the DLRE2.0 model. AUCs of DLRE and DLRE2.0 increased, when there was higher F0–1 prevalence. All radiomics models had good robustness in the independent external test cohort.
Conclusions
DLRE2.0 was the most suitable model for staging significant fibrosis while considering the balance of diagnostic accuracy and clinical practicability.
Key Points
• The non-invasive discrimination of significant fibrosis (≥ F2) in patients with chronic liver disease (CLD) is clinically critical but technically challenging.
• We aimed to develop an updated deep learning radiomics model of elastography (DLRE2.0) based on our previous DLRE model to achieve significantly improved performance in ≥ F2 evaluation.
• Our study based on 807 CLD patients and 4842 images with liver biopsy found that DLRE2.0 was the most suitable model for staging significant fibrosis while considering the balance of diagnostic accuracy and clinical practicability.





Similar content being viewed by others
Abbreviations
- 2D-SWE:
-
Two-dimensional shear-wave elastography
- ≥ F2:
-
Significant fibrosis
- ≥ F3:
-
Advanced fibrosis
- AI:
-
Artificial intelligence
- ALB:
-
Albumin
- ALT:
-
Alanine transaminase
- APRI:
-
Aspartate aminotransferase to platelet ratio index
- AST:
-
Aspartate aminotransferase
- AUC:
-
The area under the ROC curve
- BMI:
-
Body mass index
- CLD:
-
Chronic liver diseases
- CNN:
-
Convolutional neural network
- CT:
-
Computed tomography
- DB:
-
Direct bilirubin
- DLRE:
-
Deep learning radiomics model of elastography
- F4:
-
Cirrhosis
- FBG:
-
Fasting blood glucose
- FIB-4:
-
Fibrosis index based on four factors
- GGT:
-
Gamma-glutamyl transpeptidase
- IB:
-
Indirect bilirubin
- LB:
-
Liver biopsy
- MLP:
-
Multi-layer perceptron
- MR:
-
Magnetic resonance
- PLT:
-
Platelet count
- PT%:
-
Prothrombin activity percentage
- ROC:
-
Receiver operating characteristic curves
- TB:
-
Total bilirubin
- TE:
-
Transient elastography
References
GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385:117–171
Lim JK, Flamm SL, Singh S et al (2017) American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 152:1536–1543
Terrault NA, Lok AS, McMahon BJ et al (2018) Update on prevention, diagnosis, and treatment and of chronic hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 67(4):1560–1599
Ferraioli G, Wong VW-S, Castera L et al (2018) Liver ultrasound elastography: an update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol 44(12):2419–2440
Shiha G, Ibrahim A, Helmy A et al (2017) Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int 11:1–30
Terrault NA, Bzowej NH, Chang KM et al (2016) American Association for the Study of Liver D. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63:261–283
Ofliver EAF (2017) EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 67:370
Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344:495–500
Dietrich CF, Bamber J, Berzigotti A et al (2017) EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long Version). Ultraschall Med 38:e16–e47
Degos F, Perez P, Roche B et al (2010) Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 53:1013–1021
Gao Y, Zheng J, Liang P et al (2018) Liver fibrosis with two-dimensional US shear-wave elastography in participants with chronic hepatitis B: a prospective multicenter study. Radiology 289(2):407–415
Choi KJ, Jang JK, Lee SS et al (2018) Development and validation of a deep learning system for staging liver fibrosis by using contrast agent-enhanced CT images in the liver. Radiology 289(3):688–697
Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Liver fibrosis: deep convolutional neural network for staging by using gadoxetic acid-enhanced hepatobiliary phase MR images. Radiology 287:146–155
Park HJ, Lee SS, Park B et al (2019) Radiomics analysis of gadoxetic acid-enhanced MRI for staging liver fibrosis. Radiology 290:380–387
Wang K, Lu X, Zhou H et al (2019) Deep learning radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 68:729–741
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293
Liu Y, Dong CF, Yang G et al (2015) Optimal linear combination of ARFI, transient elastography and APRI for the assessment of fibrosis in chronic hepatitis B. Liver Int 35:816–825
Kim WR, Berg T, Asselah T et al (2016) Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol 64:773–780
Pan SJ, Yang Q (2009) A survey on transfer learning. IEEE Trans Knowl Data Eng 22(10):1345–1359
Popescu MC, Balas VE, Perescu-Popescu L et al (2009) Multilayer perceptron and neural networks. WSEAS Trans Circuits Syst 8(7):579–588
Xue LY, Jiang ZY, Fu TT et al (2020) Transfer learning radiomics based on multimodal ultrasound imaging for staging liver fibrosis. Eur Radiol 30(5):2973–2983
Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Deep learning for staging liver fibrosis on CT: a pilot study. Eur Radiol 28:4578–4585
Harris R, Harman DJ, Card TR, Aithal GP, Guha IN (2017) Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol 2:288–297
Bedossa P, Carrat F (2009) Liver biopsy: the best, not the gold standard. J Hepatol 50:1–3
Acknowledgements
We thank all the patients involved in this study. The authors would like to acknowledge the instrumental and technical support of multimodal biomedical imaging experimental platform, Institute of Automation, Chinese Academy of Sciences.
Funding
The work is supported by the National Natural Science Foundation of China under Grant No. 81827802, 81527805, 61231004, 61671449, and 61401462, the Chinese Academy of Sciences under Grant No. KFJ-STS-ZDTP-059, YJKYYQ20180048, QYZDJ-SSW-JSC005, and XDB32030200.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Rongqin Zheng
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Lu, X., Zhou, H., Wang, K. et al. Comparing radiomics models with different inputs for accurate diagnosis of significant fibrosis in chronic liver disease. Eur Radiol 31, 8743–8754 (2021). https://doi.org/10.1007/s00330-021-07934-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07934-6